Amsacrine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
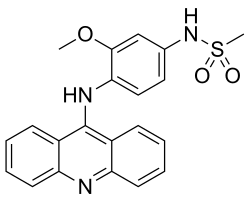
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Amsacrine | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 21 H 19 N 3 O 3 S | |||||||||||||||||||||
| Brief description |
powder |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class |
Antineoplastic |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 393.46 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
197-199 ° C |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Amsacrine (m-AMSA) is a synthetically prepared chemical compound from the group of acridine - derivatives , as cytostatically effective drug (as so-called tumor antibiotic place) use.
Clinical information
application areas
Nowadays (2019) the drug is mainly used for the therapy of acute myeloid leukemia (AML) and acute lymphatic leukemia . Amsacrin is mainly used for conditioning (HSCT) before an allogeneic stem cell transplant . Use in combination with fludarabine and cytarabine as part of the FLAMSA regimen is common here .
Therapy regimen
- FLAMSA ( fludarabine , cytarabine , amsacrine in combination with radiation of 2-12 Gy )
- FLAMSA-RIC (intensity -reduced regime in which the radiation is replaced by busulfan or treosulfan )
- ACE
unwanted effects
Pancytopenia , gastrointestinal disorders, cerebral seizures , central nervous disorders, alopecia , phlebitis , allergic skin reactions, cardiac arrhythmias, cardiac insufficiency up to cardiac arrest, liver dysfunction and eye damage can occur as undesirable effects .
Pharmacological properties
Mechanism of action
The effect of amsacrine is based on that of the intercalation in the deoxyribonucleic acid of the tumor cells. The resulting change in the structure of the DNA inhibits both DNA replication and transcription, since the activity of DNA polymerases, RNA polymerases and transcription factors is reduced. There is also an inhibition of topoisomerase II activity, which also contributes to cytotoxicity .
Absorption and distribution in the body
The elimination of amsacrine place after intravenous by administering metabolism in the liver. The half-life is 3–9 hours.
Trade names
Amsidyl (D), Amsalyo (F), Amekrin (S)
Individual evidence
- ↑ a b c d e data sheet Amsacrine hydrochloride ≥98% (TLC), powder from Sigma-Aldrich , accessed on November 27, 2017 ( PDF ).
- ↑ Christoph Schmid, Michael Schleuning, Georg Ledderose, Johanna Tischer, Hans-Jochem Kolb: Sequential Regimen of Chemotherapy, Reduced-Intensity Conditioning for Allogeneic Stem-Cell Transplantation, and Prophylactic Donor Lymphocyte Transfusion in High-Risk Acute Myeloid Leukemia and Myelodysplastic Syndrome . In: Journal of Clinical Oncology . tape 23 , no. 24 , 2005, pp. 5675-5687 , doi : 10.1200 / JCO.2005.07.061 .
- ↑ Tim Pfeiffer, Michael Schleuning, Matthias Eder, Marta Krejci, Karin Kolbe, Christof Scheid, Renate Arnold, Armin Gerbitz, Donald Bunjes, Ernst Holler, Gesine Bug, Maximillian Christopeit, Hildegard Greinix, Matthias Stelljes, Wolfgang A. Bethge, Daniel V Oruzio, Elke Dammann, Margret Rothmayer, Ralf Meyer, Jiri Mayer, Rainer Schwerdtfeger, Arnold Ganser, Hans-Jochem Kolb, Christoph Schmid: Improved Outcome for Patients with Acute Myeloid Leukemia (AML) and Myelodysplastic Syndrome (MDS) with Poor Risk Cytogenetics - Result from An Analysis on 172 Patients Receiving FLAMSA-RIC Conditioning for Allogeneic Stem Cell Transplantation (SCT) . In: Blood . tape 112 , no. 11 , 2008, p. 1971–1971 ( online - free full text).
- ↑ Kolb, Hans-Jochem, et al. "Allogeneic Stem Cell Transplantation for MDS and sAML Following Reduced Intensity Conditioning and Preemptive Donor Lymphocyte Transfusion." (2006): 324-324.
- ↑ C. Schmid, M. Schleuning, M. Hentrich, GE Markl, A. Gerbitz, J. Tischer, G. Ledderose, D. Oruzio, W. Hiddemann, H.-J. Kolb: High antileukemic efficacy of an intermediate intensity conditioning regimen for allogeneic stem cell transplantation in patients with high-risk acute myeloid leukemia in first complete remission . In: Bone Marrow Transplant . tape 41 , no. 8 , 2008, p. 721–727 , doi : 10.1038 / sj.bmt.1705965 (free full text).
- ↑ Christoph Schmid, Michael Schleuning, Rainer Schwerdtfeger, Bernd Hertenstein, Eva Mischak-Weissinger, Donald Bunjes, Stephanie v. Harsdorf, Christoph Scheid, Udo Holtick, Hildegard Greinix, Felix Keil, Barbara Schneider, Michael Sandherr, Gesine Bug, Johanna Tischer, Georg Ledderose, Michael Hallek, Wolfgang Hiddemann, Hans-Jochem Kolb: Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation . In: Blood . tape 108 , no. 3 , 2006, p. 1092-1099 , doi : 10.1182 / blood-2005-10-4165 , PMID 16551971 (free full text).
- ↑ Holtick, Udo, et al. "FLAMSA reduced-intensity conditioning is equally effective in AML patients with primary induction failure as well as in first or second complete remission." European journal of haematology 96.5 (2016): 475-482.
- ↑ Holtick, Udo, et al. "Similar outcome after allogeneic stem cell transplantation with a modified FLAMSA conditioning protocol substituting 4 Gy TBI with treosulfan in an elderly population with high-risk AML." Annals of hematology 96.3 (2017): 479-487.
- ↑ Reģionālā cancer centrum i samverkan (ed.) Acute myeloisk leukemi (AML) . 2016, ISBN 978-91-87587-53-5 ( PDF ). PDF ( Memento from December 1, 2017 in the Internet Archive )
- ↑ a b c Hasso Scholz, Ulrich Schwabe (ed.): Pocket book of drug treatment: applied pharmacology . 13th edition. Springer, Berlin / Heidelberg 2005, ISBN 3-540-20821-6 , pp. 421 .
- ^ Charlotte Niemeyer, Angelika Eggert: Pediatric Hematology and Oncology. Springer, Berlin / Heidelberg 2018, ISBN 978-3-662-43686-8 , pp. 175-185, doi: 10.1007 / 978-3-662-43686-8