Pyrrole
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
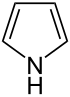
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Pyrrole | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 5 N | |||||||||||||||
| Brief description |
colorless, fiery-tasting liquid with a chloroform-like odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 67.09 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.97 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−24 ° C |
|||||||||||||||
| boiling point |
131 ° C |
|||||||||||||||
| Vapor pressure |
8.7 h Pa (20 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.5082 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
63.1 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Pyrrole (systematic name according to IUPAC : azole ) is an organic compound from the group of heteroaromatics and the parent system of pyrroles (azoles) . For example, the porphyrins , including porphine , heme and chlorophyll , vitamin B12 and the bile pigments ( bilirubin , urobilin ) are made up of pyrrole rings . The origin of the name comes from the Greek ( pyrros = fire red).
History and characteristics
Pyrrole was found and isolated in coal tar by FF Runge in 1834 . It was later found in bone tar and bone oil .
In its pure state, pyrrole is a colorless liquid with a chloroform- like odor that turns brown over time and becomes resinous in air. Pyrrole vapors turn a spruce chip moistened with hydrochloric acid red. This is a qualitative detection reaction for pyrrole and was the historical reason for the name.
Compared to the other amines, pyrrole (more precisely: 1 H -pyrrole) is only very weakly basic (pK b 13.6), because the lone pair of electrons is involved in the formation of the aromatic π-electron sextet and consequently the aromaticity when protonated on the nitrogen atom will be annulled.
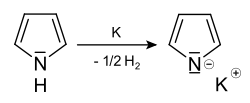
In contrast, the NH group can be deprotonated without loss of aromaticity. For example, pyrrole reacts with metallic potassium with evolution of hydrogen to pyrrole potassium (potassium pyrrolide):
Manufacturing
Industrially, pyrrole is synthesized from furan and ammonia :
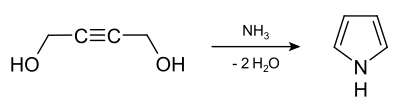
It can also be obtained from butyne-1,4-diol by heating with ammonia under pressure:
Substituted pyrroles are obtained via the Knorr pyrrole synthesis or via the Paal-Knorr synthesis from substituted 1,4-diketones, the z. B. be generated by oxidative dimerization of β-ketoesters with iodine .
Derivatives
- Tetrapyrrole
- Polypyrrole
- Iodol ( tetraiodopyrrole , C 4 I 4 NH) is formed when pyrrole is treated with potassium iodide and forms an amorphous, gray-brown, odorless powder. It is soluble in warm alcohol, ether and fatty oils, not in water, and decomposes in the light and at a temperature of 140 ° C. It was recommended as a replacement for iodoform in wound treatment, whereby its odorlessness is particularly important. First commercially produced in 1885 by Kalle oHG in Wiesbaden-Biebrich.
literature
- V. v. Judge: Organic Chemistry . Verlag Friedrich Cohen, Bonn 1913, vol. II, p. 722 (discovery)
- Beyer / Walter: Textbook of organic chemistry . 19th edition. Hirzel Verlag, Stuttgart 1981, p. 668 ff. (Detection reaction, syntheses, reactions)
- Eicher / Hauptmann: Heterocyclic Chemistry . 2nd Edition. WILEY-VCH GmbH, Weinheim 2003, pp. 86–98 (structure, physical properties, spectroscopic properties; chemical properties, syntheses; important derivatives, natural substances, drugs; use as a reagent, building block, auxiliary in organic synthesis)
Individual evidence
- ↑ a b c d Entry on pyrrole. In: Römpp Online . Georg Thieme Verlag, accessed on September 29, 2014.
- ↑ a b c d e f g Entry on pyrrole in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-25.
- ↑ Description of the substance iodol , production ( Memento from December 11, 2015 in the Internet Archive ) (PDF file; 366 kB).
- ^ Kalle company description from 1952, page 8.