Clenbuterol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
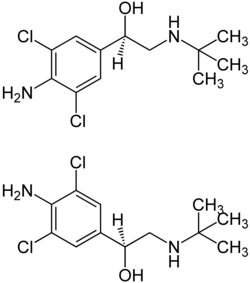
| ( R ) -Clenbuterol (top) and ( S ) -Clenbuterol (bottom), 1: 1 mixture of stereoisomers | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Clenbuterol | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula |
|
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Mechanism of action | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | ||||||||||||||||||||||
| density |
|
|||||||||||||||||||||
| Melting point |
|
|||||||||||||||||||||
| pK s value |
9.5 |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Clenbuterol is a drug from the group of β 2 sympathomimetics and is used to treat asthma . It also has a reliable tocolytic (labor-inhibiting) effect, which is used in veterinary medicine . Clenbuterol is subject to a medical prescription and is distributed by Boehringer Ingelheim Pharma KG . In addition, the substance fell into disrepute due to its misuse in sports medicine as a doping agent .
doping
Clenbuterol has come into disrepute due to its illegal use in Europe in calf fattening and its improper use as a doping agent. Prominent athletes such as the former athlete Katrin Krabbe , the winner of the Tour de France 2010 and the Giro d'Italia 2011 Alberto Contador and the former racing cyclists Dschamolidin Abduschaparov and Frank Vandenbroucke also used Clenbuterol to improve performance, as did the bodybuilder Andreas Münzer and the Australian heavyweight boxer Lucas Browne .
Although Clenbuterol does not belong to the group of anabolic steroids , an anabolic effect on the striated muscles is discussed. Since 2012, Clenbuterol has also been used (not only by bodybuilders) as a means to burn body fat faster.
Clenbuterol can cause a number of side effects, such as an increased heart rate ( tachycardia ) or muscle tremors (subtle tremor ) as well as a slight increase in body temperature and headaches. Most of these side effects are temporary and usually go away if you continue to use them. Although Clenbuterol is used in sport to increase performance, the potential for side effects in healthy people is significantly lower than when taking hormone preparations. However, typical side effects of anabolic hormones are not to be expected when taking Clenbuterol, as Clenbuterol does not interfere with the sensitive pituitary - gonadal hormonal system. The Poison Control Center in Sydney had analyzed the 63 cases treated at the center. It found that cases have increased significantly since 2012, with 84% of them being hospitalized, of which there was one death. The most common side effects include a drop in blood pressure, sinus tachycardia , significantly increased levels of cardiac troponin (403 ng / L (reference interval [RI], <14 ng / L) and a drop in potassium levels. The authors point out that Low-dose Clenbuterol, as it can be ingested with the diet of calves fattened with Clenbuterol, does not pose a problem in the clinic, but the high doses can be life-threatening due to weight loss and muscle gain.
chemistry
Clenbuterol is a chiral drug with a stereocenter . The active isomer ( eutomer ) is ( R ) -clenbuterol. The racemate , the 1: 1 mixture of the ( S ) and ( R ) isomers, is used therapeutically .
Analytics
The reliable identification and quantitative determination of clenbuterol in urine or blood samples is carried out with the aid of gas chromatography-mass spectrometry coupling ( GC-MS ).
Trade names
Spiropent (D) - tablets and drops
- Combination preparations
- with ambroxol
- Mucospas (D, A), Spasmo-Mucosolvan (D)
Veterinary medicine
Venti Plus (A), Ventipulmin (A)
Individual evidence
- ↑ Carsten Culmseea, Vera Junkera, Serge Thalc, Wolfram Kremersa, Sandra Maiera, Harald Jörn Schneider, Nikolaus Plesnilac, Josef Krieglstein: Enantio-selective effects of clenbuterol in cultured neurons and astrocytes, and in a mouse model of cerebral ischemia . In: European Journal of Pharmacology . tape 575 , no. 1 , 2007, p. 57-65 , doi : 10.1016 / j.ejphar.2007.07.066 .
- ↑ A. Carpy, J.-M. Leger, J.-C. Colleter: Chlorhydrate d '(amino-4 dichloro-3,5 phenyl) -1 tert-butylamino-2 ethanol (Clenbutérol, NAB-365) . In: Acta crystallographica. Section B, Structural crystallography and crystal chemistry . tape 36 , no. 11 , 1980, pp. 2837-2840 , doi : 10.1107 / S0567740880010230 .
- ↑ Patent DE2157040 : New process for the production of 4-amino-3,5-dihalo-phenyl-ethanolamines. Published May 24, 1973 Applicant: Dr. Karl Thomae GmbH, inventor: Johannes Keck, Axel Prox.
- ^ The Merck Index : An Encyclopedia of Chemicals, Drugs, and Biologicals. 14th edition. Merck & Co., Inc., Whitehouse Station, NJ, USA 2006, ISBN 0-911910-00-X , p. 393.
- ↑ Entry on Clenbuterol. In: Römpp Online . Georg Thieme Verlag, accessed on June 28, 2019.
- ↑ a b Data sheet Clenbuterol hydrochloride from Sigma-Aldrich , accessed on March 23, 2011 ( PDF ).
- ↑ Entry on Clenbuterol in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ UCI press release ( Memento of the original of October 22, 2010 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Tages-Anzeiger "Positive doping test at Contador" .
- ↑ Boxing: Conquerors of Chagaev tested positive for doping. In: SPIEGEL ONLINE. Retrieved March 22, 2016 .
- ↑ MAE Systems GmbH | Pd: Lucas Browne tested positive. (No longer available online.) In: BOXEN HEUTE | News, results, tickets, dates, videos. Archived from the original on March 25, 2016 ; accessed on March 22, 2016 . Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Jonathan Brett, Andrew H Dawson & Jared A Brown (2014). Clenbuterol toxicity: a NSW Poisons Information Center experience. Med J Aust 2014; 200 (4): 219-221.
- ↑ A. González-Antuña, P. Rodríguez-González, I. Lavandera, G. Centineo, V. Gotor, JI García Alonso: Development of a routine method for the simultaneous confirmation and determination of clenbuterol in urine by minimal labeling isotope pattern deconvolution and GC-EI-MS. In: Anal Bioanal Chem. 2012 Feb; 402 (5), pp. 1879-1888. PMID 22241580 .
- ↑ A. Thomas, H. Geyer, W. Schänzer, C. Crone, M. Kellmann, T. Moehring, M. Thevis: Sensitive determination of prohibited drugs in dried blood spots (DBS) for doping controls by means of a benchtop quadrupole / Orbitrap mass spectrometer. In: Anal Bioanal Chem. 2012 Jan 10. PMID 22231507 .
literature
- Andreas Gleixner: Pharmacological studies on the accumulation of Clenbuterol and steroidal anabolic steroids in pigmented tissues . Herbert Utz Verlag, Munich 1996, ISBN 3-89675-109-3 .
Web links
- Entry on Clenbuterol at Vetpharm, accessed November 22, 2011.