Cyclopropanecarbonitrile
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
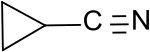
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Cyclopropanecarbonitrile | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 5 N | |||||||||||||||
| Brief description |
clear colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 67.09 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density | ||||||||||||||||
| boiling point |
135 ° C |
|||||||||||||||
| solubility |
miscible with water, soluble in diethyl ether and n-hexane |
|||||||||||||||
| Refractive index |
1.4229 (20 ° C, 589 nm) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Cyclopropanecarbonitrile is the smallest cycloalkane with a nitrile group linked directly to the cyclopropyl group . Because of its easy synthetic accessibility, it is suitable as a starting compound for functional cyclopropane derivatives, such as. B. Cyclopropanecarboxylic acid or cyclopropylamine and for ring-open unsaturated C 3 -nitriles, such as the isomers crotononitrile and allyl cyanide .
presentation
The preparation of cyclopropanecarbonitrile by distilling 4-chlorobutyronitrile with potassium hydroxide was first described in 1898.
4-chlorobutyronitrile is obtained by reacting 4-chloro-1-bromopropane with potassium cyanide in 60 to 70% yield, this in turn by reacting 3-chloro-1-propanol with phosphorus tribromide in 94% yield. 3-Chloro-1-propanol (trimethylene chlorohydrin) is produced according to a specification by Carl S. Marvel when hydrogen chloride gas is passed through 1,3-propanediol at 160 ° C. in a yield of 50 to 60%.
The ring closure to cyclopropane can be carried out with sodium amide in liquid ammonia with 60% or 89% yield, with sodium hydroxide NaOH in dimethyl sulfoxide DMSO (100% yield) or without a solvent under phase transfer catalysis with solid NaOH and benzyltriethylammonium chloride (99% yield).
An alternative synthesis route leads from cyclopropanecarbaldehyde via the oxime formed with hydroxylamine by elimination of water with concentrated formic acid to cyclopropanecarbonitrile in yields of over 90%.
properties
Cyclopropanecarbonitrile is a clear, colorless liquid at room temperature that mixes with water and some organic solvents.
When heated to temperatures of 660-760 ° K at pressures of 2-89 Torr, cyclopropanecarbonitrile isomerizes, with the opening of the cyclopropane ring under tension , predominantly to the trans -isomeric crotononitrile in addition to a little allyl cyanide and traces of methacrylonitrile .
Applications
From cyclopropanecarbonitrile some derivatives are accessible by classical methods of organic chemistry, the starting materials u. a. for pharmaceuticals and pesticides supply.
The result is by catalytic or enzymatic hydration cyclopropanecarboxamide (A) obtained by Hofmann rearrangement using sodium hypochlorite in cyclopropylamine can be converted (B), a block for the fluoroquinolone - antibiotic ciprofloxacin , which larvicide cyromazine or herbicide safeners Cyprosulfanid.
The reaction with methyl magnesium chloride provides cyclopropyl methyl ketimine (C), which reacts with water to form cyclopropyl methyl ketone (D), a building block for the fungicide cyprodinil .
Catalytic hydrogenation of cyclopropyl cyanide leads to cyclopropylmethylamine (E), a building block for the herbicide cyclopyranil.
The complete hydrolysis of the nitrile group of the cyclopropanecarbonitrile with sulfuric acid or with enzyme preparations from Rhodococcus strains yields cyclopropanecarboxylic acid, a component for the herbicides cyprazole, cypromid and cyclosulfamuron and the fungicide cyprofuram.
Individual evidence
- ↑ a b c d e data sheet cyclopropanecarbonitrile from Sigma-Aldrich , accessed on January 25, 2018 ( PDF ).
- ^ A b c William M. Haynes: CRC Handbook of Chemistry and Physics, 97th Edition . CRC Press, Boca Raton, FL, USA 2017, ISBN 978-1-4987-5429-3 , pp. 3-138 .
- ↑ Data sheet Cyclopropanecarbonitrile at AlfaAesar, accessed on January 25, 2018 ( PDF )(JavaScript required) .
- ↑ a b c J.B. Cloke, RJ Anderson, J. Lachmann, GE Smith: The preparation of cyclopropyl cyanide and trimethylene chlorobromide . In: J. Am. Chem. Soc. tape 53 , no. 7 , 1931, pp. 2791–2796 , doi : 10.1021 / ja01358a054 .
- ↑ CFH Allen: γ-Chlorobutyronitrile In: Organic Syntheses . 8, 1928, p. 52, doi : 10.15227 / orgsyn.008.0052 ; Coll. Vol. 1, 1941, p. 156 ( PDF ).
- ↑ CS Marvel, HO Calvery: trimethylene chlorohydrin In: Organic Synthesis . 8, 1941, p. 112, doi : 10.15227 / orgsyn.008.0102 ; Coll. Vol. 1, 1941, p. 533 ( PDF ).
- ↑ MJ Schlatter: cyclopropyl cyanide in: Organic Syntheses . 23, 1943, p. 20, doi : 10.15227 / orgsyn.023.0020 ; Coll. Vol. 3, 1955, p. 223 ( PDF ).
- ↑ Patent US3843709 : Preparation of cyclopropyl cyanide from 4-chlorobutyronitrile. Applied October 15, 1973 , published October 22, 1974 , Applicant: Gulf Research & Development Co., Inventor: JD Bacha, CM Selwitz.
- ↑ Patent US3974199 : Process for production of cyclopropylcyanide. Filed June 16, 1975 , published August 10, 1976 , applicant: The Dow Chemical Co., inventor: JH Plonka, RG Pews.
- ↑ Patent US6222058B1 : Process for the manufacture of cyclopropanecarbonitrile. Applied March 30, 1998 , published April 24, 2001 , Applicant: Eastman Chemical Co., Inventor: S. Liang.
- ↑ DA Luckraft, PJ Johnson: Kinetics of the reactions of cyclopropane derivatives. III. Gas-phase unimolecular isomerization of cyclopropyl cyanide to the cyclopropenes . In: Int. J. Chem. Kinet. tape 5 , no. 1 , 1973, p. 137-147 , doi : 10.1002 / kin.550050112 .
- ↑ K.-T. Liu, M.-H. Shih, H.-W. Huang, C.-J. Hu: Catalytic hydration of nitriles to amides with manganese dioxide on silica gel . In: Synthesis . tape 9 , 1988, pp. 715-717 , doi : 10.1055 / s-1988-27684 .
- ↑ T. Nagasawa, H. Nanba, K. Ryuno, K. Takeuchi, H. Yamada: Nitrile hydratase of Pseudomonas chlororaphis B23 - Purification and characterization . In: The FEBS Journal . tape 162 , no. 3 , 1987, pp. 691-698 , doi : 10.1111 / j.1432-1033.1987.tb10692.x .
- ↑ Patent US4590292 : Process for the manufacture of cyclopropylamine. Filed June 10, 1986 , published May 20, 1986 , Applicant: Ciba-Geigy Corp., Inventor: JT Blackwell, HL Daughety, HC Grace, WH Oliver.
- ↑ Patent DE2061035 : Process for the production of cyclopropylmethylalkylamines. Applied on December 11, 1970 , published March 2, 1972 , Applicant: Esso Research and Engineering Co., Inventors: J. Lindner, LL Moravetz, GN Schmit, NF Newman.
- ^ CM McCloskey, GH Coleman: Cyclopropanecarboxylic acid In: Organic Syntheses . 24, 1944, p. 36, doi : 10.15227 / orgsyn.024.0036 ; Coll. Vol. 3, 1955, p. 221 ( PDF ).
- ↑ MA Cohen, J. Sawden, NJ Turner: Selective hydrolysis of nitriles under mild conditions by on enzymes . In: Tetrahedron Lett. tape 31 , no. 49 , 1990, pp. 7223-7226 , doi : 10.1016 / S0040-4039 (00) 97285-X .
- ↑ Compendium of Pesticide Common Names alanwood.net (English).





