Dimethylacetyl succinate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
|
Mixture of stereoisomers (mixture of two isomers) Structural formula without specifying the stereochemistry |
||||||||||||||||
| General | ||||||||||||||||
| Surname | Dimethylacetyl succinate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 8 H 12 O 5 | |||||||||||||||
| Brief description |
white crystal powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 188.18 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density | ||||||||||||||||
| Melting point | ||||||||||||||||
| boiling point | ||||||||||||||||
| solubility |
soluble in water (56.2 g l −1 at 20 ° C ), in methanol and toluene |
|||||||||||||||
| Refractive index |
1.435-1437 (20 ° C, 589 nm) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Dimethylacetyl succinate is a derivative of succinic acid , which is present as dimethyl succinate with an acetyl group in the 2-position. The compound is a starting material for heterocycles , e.g. B. Pyrazolones as in the food coloring tartrazine .
Occurrence and representation
Dimethylacetylsuccinate can be obtained analogously to the Organic Syntheses specification for diethyl acetylsuccinate by reacting methyl acetoacetate with methyl chloroacetate .
This synthesis was published in 1877 by Max Conrad for the diethylacetylsuccinic acid diethyl ester (Diethylacetylsuccinat DEAS) known as "Acetsuccinsäureäthylester".
Radical addition of acetaldehyde to the double bond of maleic acid dimethyl ester is supposed to deliver DMAS in high yield and purity in a continuous process.
properties
Dimethylacetyl succinate is a white crystal powder that, due to its low melting temperature, can also be present as a clear liquid at room temperature. The substance dissolves relatively well in water, as well as in methanol and toluene .
Applications
Dimethylacetyl succinate is a suitable starting compound for cyclobutyl methyl ketone
in which a ketal-protected 1,4-dihalogen compound is reductively cyclized with lithium naphthalenide to form the four-membered cyclobutane ring .
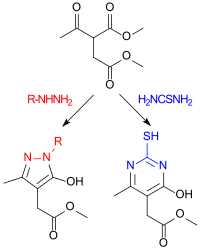
Dimethylacetyl succinate reacts with alkyl and aryl hydrazines to give pyrazolones and with thiourea to give 4-pyrimidinones , which are of interest as heterocyclic acetic acid derivatives .
The hydroxyl groups on the pyrazole or pyrimidine ring can easily be exchanged with phosphorus oxychloride POCl 3 for a chlorine atom, which can more easily be replaced by other substituents.
The β-ketoester function in the acetylsuccinic acid dimethyl ester is accessible to a Japp-Klingemann reaction with diazonium salts to give aryl hydrazones, from which dihydropyrazolones or the pyrazolols which are tautomeric to them are formed by cyclization .
Under controlled conditions (approx. 20 ° C, pH 8), initially from phenyldiazonium chloride (R 1 = phenyl) and dimethylacetylsuccinate with elimination of the acetate group, dimethylphenylhydrazone succinate is formed, which when heated to 60 ° C and addition of ammonia at pH 8.4 in 1 -Phenyl-3-carbomethoxy-5-pyrazole passes over in 94% yield and high purity and can be isolated by acidification. Reaction with further aryldiazonium chloride gives the corresponding azo dye from the class of tartrazines.
Today's most common industrial process for the production of the lemon-yellow food dye tartrazine , also known as Acid Yellow 23 or E 102 , which was originally used as a yellow, light-resistant wool dye, is based on the simplified one-step synthesis route .
Double the amount of diazotized sulfanilic acid is added to DMAS (or diethylacetyl succinate DEAS) in an alkaline medium and, after cyclization and renewed diazotization, the ester group is hydrolyzed to the very water-soluble carboxylic acid salt by heating in sodium hydroxide solution.
Individual evidence
- ↑ a b c Entry on Dimethyl Acetylsuccinate at TCI Europe, accessed on April 25, 2019.
- ↑ a b c d DMAS Dimethylacetyl Succinate. ESIM Chemicals GmbH, September 2015, accessed on April 25, 2019 .
- ↑ a b c d e f data sheet dimethylacetyl succinate from Sigma-Aldrich , accessed on April 25, 2019 ( PDF ).
- ↑ a b c G.W. Craig, M. Eberle, C. Lamberth, T. Vettiger: Dimethyl acetylsuccinate as a versatile synthon in heterocyclic chemistry - A facile synthesis of heterocyclic acetic acid derivatives . In: J. Prakt. Chem. Volume 342 , no. 5 , 2000, pp. 504-507 , doi : 10.1002 / 1521-3897 (200006) 342: 5 <504 :: AID-PRAC504> 3.0.CO; 2-R .
- ↑ H. Adkins, N. Isbell, B. Wojcik: Ethyl Acetosuccinate In: Organic Syntheses . 14, 1934, p. 38, doi : 10.15227 / orgsyn.014.0038 ; Coll. Vol. 2, 1943, p. 262 ( PDF ).
- ^ M. Conrad: XLI. Acetsuccinic acid ester and its derivatives . In: Justus Liebigs Ann. Chem. Band 188 , no. 1-2 , 1887, pp. 217-226 , doi : 10.1002 / jlac.18871880111 .
- ↑ Patent CN102311345A : Continuous synthesis method of dimethyl acetylsuccinate. Filed September 9, 2009 , published January 11, 2012 , applicant: Hebei Hua Chem Group Co. Ltd., inventor: Q. Ren, J. Ge, G. Zhang, F. Zhang, S. Wang, X. Fan ,
- ↑ K. Ramig, Y. Dong, SD Van Arnum: A convenient preparation of cyclobutyl ketones: Naphthalene-catalyzed reductive cyclization of substituted 1,4-dihalobutanes . In: Tetrahedron Lett. tape 37 , no. 4 , 1996, pp. 443-446 , doi : 10.1016 / 0040-4039 (95) 02194-9 .
- ↑ J.-W. Gao: Lithium Naphthalenide . In: Synlett . tape 23 , 2012, p. 317-318 , doi : 10.1055 / s-0031-1290127 .
- ↑ Patent US4837335 : Process for the preparation of pure 1-phenyl-3-carbalkoxy-5-hydroxypyrazoles. Registered on April 4, 1988 , published on June 6, 1989 , applicant: Chemie Linz GmbH, inventor: H. Stückler, W. Dobramysl.
- ^ JH Ziegler, M. Locher: Ueber die Tartrazine, a new class of dyes . In: Ber. German Chem. Ges. Volume 20 , no. 1 , 1887, p. 834-840 , doi : 10.1002 / cber.188702001188 .
- ↑ Patent US2457823 : Production of pyrazolone azo dyes. Applied November 29, 1945 , published January 4, 1949 , applicant: Ilford Ltd., inventor: JD Kendall, DJ Fry.