Dioxacarb
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
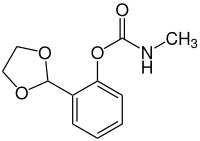
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Dioxacarb | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 11 H 13 NO 4 | ||||||||||||||||||
| Brief description |
colorless, crystalline solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 223.23 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.46 g cm −3 |
||||||||||||||||||
| Melting point |
114 ° C |
||||||||||||||||||
| boiling point |
decomposes when heated |
||||||||||||||||||
| Vapor pressure |
0.04 · 10 −3 Pa (20 ° C) |
||||||||||||||||||
| solubility | |||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Dioxacarb is a synthetic insecticide from the active ingredient group of carbamates . It was launched in 1968 by the Ciba company .
presentation
Salicylaldehyde is reacted with ethylene glycol . The resulting 2- (1,3-dioxolan-2-yl) phenol is reacted with methyl isocyanate to form dioxacarb.
properties
Dioxacarb is a white, crystalline solid. It is sparingly soluble in water and decomposes when heated. It also degrades quickly in both acidic and alkaline environments. With a half-life of two days, it is not persistent in the soil.
Use and mode of action
Dioxacarb can be used to control a wide range of insects. So it works against cicadas in rice cultivation, as well as against aphids , various beetles and cockroaches in the household. It can also be used particularly well against Colorado beetles and the alfalfa beetle . It is less effective against Lepidoptera and Diptera . In addition, it has no acaricidal effect.
Dioxacarb acts as a contact and feeding poison and makes insects unable to move shortly after contact ( knock-down effect). As with all carbamate insecticides, the mode of action is based on the inhibition of the enzyme acetylcholine esterase in the synapses of the nervous system . This causes the nerves to stop transmitting stimuli, which can lead to paralysis or even respiratory failure and ultimately death.
Admission
Plant protection products containing dioxacarb are not permitted in either the European Union or Switzerland . In the EU, the maximum residue limit in all foods is 0.01 mg / kg.
Individual evidence
- ↑ a b c d e f g Entry on Dioxacarb in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire , accessed on February 2, 2019.
- ↑ a b c d e f Entry on dioxacarb in the GESTIS substance database of the IFA , accessed on February 4, 2019 (JavaScript required)
- ↑ Entry on Dioxacarb in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 4, 2019. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ LGC Standards GmbH: Safety data sheet . (PDF) In: www.lgcstandards.com. January 14, 2016, accessed February 4, 2019 .
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-8155-1853-6 , pp. 79 ( limited preview in Google Book search).
- ^ A b Hayes, Wayland J. ,, Laws, Edward R.,: Handbook of pesticide toxicology. Volume 3, Classes of pesticides . San Diego, California, ISBN 978-1-4832-8863-5 ( limited preview in Google Book Search [accessed February 5, 2019]).
- ↑ a b MacBean, C. (Colin), British Crop Protection Council .: The pesticide manual: a world compendium. Sixteenth ed. Alton, Hampshire, ISBN 978-1-901396-86-7 .
- ↑ Richard Wegler: Chemistry of the pesticides and pesticides . Springer Science & Business Media, 2012, ISBN 978-3-642-46210-8 ( limited preview in Google book search).
- ^ Directorate-General for Health and Food Safety of the European Commission: Entry on Dioxacarb in the EU pesticide database ; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on February 20, 2019.

