Fenitrothion
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Fenitrothion | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 9 H 12 NO 5 PS | |||||||||||||||
| Brief description |
oily, yellowish liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 277.25 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.33 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
3.4 ° C |
|||||||||||||||
| boiling point |
140–145 ° C (at 0.1 hPa) |
|||||||||||||||
| Vapor pressure |
0.18 Pa (20 ° C) |
|||||||||||||||
| solubility |
almost insoluble in water (21 mg l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Fenitrothion was launched in 1959 by Sumitomo Chemical Company and Bayer AG . Fenitrothion is significantly less toxic than parathion with a similar spectrum of activity and a structure so similar that it can be produced in the same facilities. Fenitrothion is used as an effective and widely used contact insecticide and selective acaricide .
properties
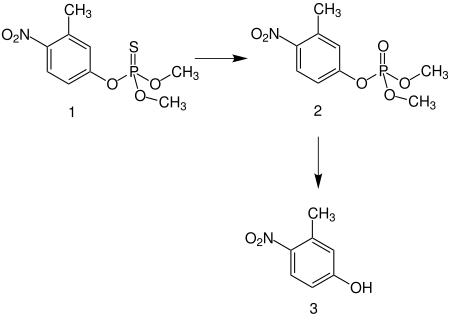
Fenitrothion is an oily, yellowish liquid with a characteristic odor. Chemically, it belongs to the group of thiophosphoric acid esters . In the target organism, fenitrothion ( 1 ) is converted into fenitrooxone ( 2 ), the actual active ingredient, via oxidative desulfurization :
( 2 ) is further metabolized to 3-methyl-4-nitrophenol ( 3 ).
Admission
The use of fenitrothion is banned in the EU. In Switzerland it was contained as an active ingredient in two preparations that were used against various harmful insects, aphids and mites in orchards and viticulture. The approval of these preparations has since been withdrawn.
safety instructions
Fenitrothion is a contact poison which , like all organophosphates, acts as a neurotoxin in humans .
In the studies carried out so far, there was no evidence of a teratogenic or reproductive toxicity. Also mutagenic properties or carcinogenic effect could not be detected.
The permitted daily dose is 0.005 and the acute reference dose 0.013 milligrams per kilogram of body weight and day.
Individual evidence
- ↑ a b c d e f g h Entry on fenitrothion in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Entry on fenitrothion in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Data sheet Fenitrothion at Sigma-Aldrich , accessed on May 14, 2017 ( PDF ).
- ↑ Entry on Fenitrothion. In: Römpp Online . Georg Thieme Verlag, accessed on August 12, 2016.
- ↑ a b Directorate-General for Health and Food Safety of the European Commission: Entry on fenitrothion in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 11, 2016.