Bat pair
| Bat pair | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|

Bat pair in Cape Vidal, Northern Valley |
||||||||||
| Systematics | ||||||||||
|
||||||||||
| Scientific name of the genus | ||||||||||
| Macheiramphus | ||||||||||
| Bonaparte , 1850 | ||||||||||
| Scientific name of the species | ||||||||||
| Macheiramphus alcinus | ||||||||||
| Bonaparte, 1850 |
The bat orb ( Macheiramphus alcinus ) is a bird of prey from the hawk family . The geographically strongly separated distribution areas include central and western Africa south of the Sahara, small areas of Madagascar, the Malay Peninsula and large parts of Indonesia as well as southeast New Guinea . The species inhabits evergreen tropical forests. The animals feed mainly on bats and small birds, as one of only a few birds of prey, the bat pair hunts after dusk. In large parts of the range, bat aqua do not breed at fixed seasons, the breeding season largely depends on the food available.
The bat pair is the only representative of the genus Macheiramphus , whose systematic position within the hawk-like species was unclear for a long time. The closest relatives are by DNA analysis of Würgadler ( Morphnus guianensis ) which Harpyie ( Harpia harpya ) and the Papua Adler ( Harpyopsis novaeguineae ). The stock is currently not considered endangered.
features
Appearance and build
The bat male is a medium-sized bird of prey with a falcon-like shape, a narrow, small beak, large eyes, a rather short tail and a small head. The nominate form M. alcinus alcinus shows a slight reverse sex dimorphism ; Females grow 6% larger than males on average.

The weight of adult birds is usually between 600 and 650 g, the body length between 41 and 51 cm. The wing length is 371 to 412 mm and the wingspan 95-120 cm, while the tail measures 166-184 mm. The comparatively long wing bones with a mean cubic length of 124 mm are striking . The beak is not particularly strong and short, from the wax skin to the beak ridge it is only 16-17 mm long. Despite the short beak, the species has very long jaws, the corner of the beak ends behind the eyes. This allows the bird to devour its prey as a whole. The eyes are relatively large for birds of prey (diameter of the cornea : 16 mm, diameter of the eyeball : 28 mm). This impression is reinforced by the plumage and the pupils, which dilate at dusk and the yellow iris almost completely disappears. The tarsometatarsus has a length of 58 to 64 mm, the middle toe including the claw measures 55–58 mm.
The hair on the back of the head and the white rims of the eyes are striking. The entire top is black or dark brown. Dark bands are usually very indistinct due to the lack of contrast. On the throat, the plumage usually has a white spot, which is divided by a narrow black stripe in the middle. The belly and chest are drawn variably: some birds have white spots there too, while others are entirely speckled with white and brown. The wax skin of adult birds is gray-blue, the legs are bluish white.
Juvenile and immature birds show plumage similar to that of adult birds, but their plumage shows more white on the underside. They also have a chest band of brown speckles, which distinguishes them from colored birds. On the tail feathers and hand feathers, which are rather gray compared to adult birds, a black banding is more evident. The iris of young birds is initially brown before gradually changing color to yellow.
Flight image
In flight, the bat pair resembles medium-sized falcons : it sails on long, wide wings, but with slightly rounded tips and a clearly bent wrist, the protruding wrist of which is clearly visible. The wingspan is about 2.3 times the body length in flight. The rectangular, rather short tail and also the slender head, the large eyes and the small beak set the bat pair apart from falcons that are very similar in flight. However, in poor lighting conditions it can be confused with some species of falcon, for example with the dark morphs of the eleonor's falcon ( Falco eleonorae ) and the slate falcon ( Falco concolor ), which occur in Africa during the winter months and the migration period, but do not reach the size of the bat's hair .
The most obvious difference is in the flight behavior: the bat pair has a slow, sluggish wing beat, which is more deliberate and deeper than that of most falcons. This flight is only occasionally interrupted by fast, powerful flapping of its wings when the bat pair encounters prey.
Vocalizations
The species is largely mute away from the breeding ground. The warning call, a weak kek-kek-kek… , is reminiscent of falcons. Apart from that, the calls are rather quiet and can be heard during courtship flight or in breeding pairs at dusk. This is a high kwik-kwik-kwik-kwik or a fast, higher kwiep of the male, which is uttered especially shortly before the start of the hunt . There is also a soft, melodious chuk-chik-chuk call and a soft wuut-wuut-wuut . The latter is similar to a short quit or qwhuit , the first part of which is whistling and the second part is rising. The bat pair threatens potential nest predators or hating birds.
Observations from New Guinea contradict the classification of the bat eel as a bird that is generally not very loud and loud , where reports of bat eel eager to call are reported. The birds called especially at dawn, in the late afternoon and during courtship with a series of five kie calls, similar to those of other hawks .
Spreading and migrations
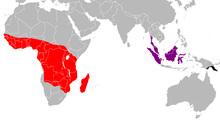
The strongly disjointed distribution area includes the central and western Africa south of the Sahara, small areas of Madagascar, the Malay Peninsula and large parts of Indonesia as well as the southeast of New Guinea . In sub-Saharan Africa, distribution gaps exist mainly in dry areas, the Horn of Africa , as well as in southern Namibia , Botswana and South Africa . In Madagascar , the distribution is limited to a few, small-scale areas near the coast. In Southeast Asia the species is native to the entire Malay Peninsula as well as Sumatra , Bangka , Natuna and Sulawesi , although it is unclear whether the latter island belongs to the breeding area; on Borneo it is absent in the interior of the island and in the northwest.
The New Guinea distribution area covers only part of the Owen Stanley Mountains in the southeast of the island. In the north it is bounded by the river Kumusi , in the south it extends to Port Moresby .
In general, the bat pair is considered to be a resident bird in its range. However, larger numbers of individuals in southwestern Madagascar in the southern winter and sightings of individual birds outside of the range in southern and eastern Africa are interpreted as evidence of a certain migration behavior. In addition, migration from higher altitudes into valleys and into the nearby lowlands was ascertained, which apparently corresponds to comparable migration movements of bats.
habitat

As a tree breeder, the bat pair is dependent on trees that are suitable for breeding. At the same time, however, he also needs sufficiently large open spaces for hunting, which enable him to maneuver in flight. A third location factor are bat roosts and hunting grounds not too far away.
The range of populated habitats includes mainly moist, lowland forest edges, river valleys or hill country interspersed with trees. The bat pair is rather rare in dry bushland. The species is apparently tolerant of encroachments on its habitat: cleared islands, heavily thinned forests, cultural and even urban landscapes such as village squares with tree cover, urban parks, tree populations along railway tracks or suburban settlements are populated.
Originally, the main hunting grounds were bodies of water, over which insects and thus bats collect more frequently at dusk. For this reason, gallery forests and other forms of vegetation along rivers are evidently preferred in large parts of the distribution area. In urban surroundings, however, the bat pair also uses street lamps or track lighting as a point of attraction for bats. Bat roosts in the form of caves, disused mine shafts, buildings or baobab trees can be found in a large number of habitats and therefore only marginally limit the range of possible habitats. The species has been proven up to an altitude of 2000 m.
Way of life
Hunting and feeding
During the day, the male bat stays upright in the branches of trees at the edge of the forest or in the nest, even outside the breeding season. He has closed his white eyelids, and the plumage around the eyes may be used to simulate open eyes and scare off potential predators.
The bat pair begins to brush its plumage for about 20-30 minutes at sunset. If he spies a potential prey, he immediately sets off from his resting position for a hunting flight, otherwise he patrols over water surfaces over which bats hunt insects at night. The hunt usually takes place at dusk, but with artificial light sources it can drag on into the night and until dawn. As soon as it has identified a prey, it accelerates its flight and then usually encounters the prey from behind and above. He then brings it to the head and devours it as a whole while in flight, which takes about 3–8 seconds. If the prey is too big to swallow in one piece, the bat male carries it to his control room to consume it there. The success rate in hunting is around 50%. A large part of the bats caught is dropped again, for example when they are too big for him.

The majority of the prey is caught within 15–30 minutes after sunset or at dawn. The number of bats captured is between four and eleven, with an average of seven animals, which are beaten at intervals of one to four minutes. In exceptional cases, the bat pair catches up to 17 bats. The daily requirement for food is 50–60 g. The relatively short duration of the hunt and the rapid consumption of the prey are probably due to the rapid loss of daylight that the bird relies on for food. Bats provide a large part of the food, but depending on availability, the bat pair also kills insects and smaller songbirds such as sand martins , salangans of the genera Collocalia and Aerodramus , night swallows and other nocturnal and crepuscular birds that are similar to bats in terms of size and habitat. The prey usually has a weight of 20–75 g and includes animals up to the size of an emerald cuckoo ( Chrysococcyx cupreus ).
The choice of prey is largely opportunistic; Bat hairs do not appear to prefer a specific species, but rather use a wide range of bats, birds and insects.
Social behavior
Bat hairs are usually observed individually away from the nest. Occasionally, however, couples or families hunt flocks of bats together from a boy and the parents.
pairing
Courtship flights are the only activity of the birds during the day that is worth mentioning. During courtship flight, birds of both sexes circle around tree height or lower, accompanied by high, high-pitched calls. Sometimes the female rolls on her back so that the claws of both birds touch each other as they fly by. In addition, the courting couple performs somersaults, quick hunting maneuvers or rolls and thrusts through the branches of the nesting tree. The male occasionally falls almost vertically from heights of up to 300 m. In flight, the female often breaks smaller branches out of the nesting tree with her claws, which she then nibbles on and which she carries with her for a while in flight and in the waiting room, before dropping them again. The male also carries out this ritualized search for nesting material, but very quickly drops the branches again. Mating is initiated by the female by perching next to the resting male. The subsequent copulation takes about 15 seconds.
Brood
The breeding season in Southeast Asia is between April and September. In western Africa, the bat pair breeds between March and June and from October to January. In East Africa, the breeding season is between April and August. With decreasing geographical latitude, it tends to take place later in the year: the main breeding season in southern Africa is between September and December. However, broods have also been observed at intervals of 10 to 11 months in a breeding pair from Zimbabwe that could use abundant food sources. This suggests that the breeding seasons of the bat pair are less related to the season than to the food supply. On the one hand, the littering time of the majority of bats plays a role, since pregnant and suckling females and the young are easy for the bat pair to capture and represent a rich source of food. On the other hand, bat hairs often lay before the start of the rainy season in order to take advantage of the higher occurrence of insects and thus also of bats.
The nest is built by both males and females in 30 to 50 days, with the male doing most of the work. It consists of thick branches and is laid out with smaller, broken branches, sometimes with green leaves. The nest measures 50–100 cm in diameter, 30–50 cm in depth and is built in Zwieseln or on outer side branches at a height of 10–60 m. A tree with light-colored bark (such as Khaya ssp. ) Is often chosen as the nest tree, which could be related to better visibility at dusk. Occasionally, trees are also used for nesting within built-up areas. In southern Africa, a large number of nests are built on the north side of the crowns. On the one hand, an attempt is made to explain this with the position of the sun in the evening, where bats are easier to spot in the north-south axis. On the other hand, some authors also point to the seasonal benefits of a north-facing nest that offers more shade in hot summer. Brood partners often remain loyal for years and occupy the same nest and territory for several years.
The clutch usually comprises one egg, and in urban surroundings more often two eggs of light, green-blue or violet color and sometimes brown speckles, measuring about 61-65 × 45-47 mm. The incubation is carried out by males and females in equal parts; the male is less involved in feeding the nestlings. The nestlings hatch after about 50 days and fly out after about 70 days, which is comparatively long for a bird of prey with a total of 120 days from egg-laying to flying out. After flying out, the young birds are still dependent on their parents for 60–80 days.
If a brood fails at an early stage, another egg is laid. However, replacement broods have also been observed after the death of a nestling and at the same time a high level of feeding. A couple in Zimbabwe raised an average of 0.7 cubs per brood, the success rate across Africa is around 0.8 cubs per brood. This low reproduction rate is due to the small size of the clutch and the losses caused by nest robbers as well as the lack of stability of the nest, which can easily collapse in the wind.
Taxonomy and research history
The bat's scientific name caused confusion for several decades. In 1850 the species was set up by Charles Lucien Bonaparte under the name Macheiramphus alcinus . A year later, Gerardus Frederik Westerman described the same species as Machaeramphus alcinus . Since Westerman's description was later incorrectly post-dated to 1848, Machaeramphus was the valid name of the genus until the middle of the 20th century. Only Herbert Girton Deignan pointed out this mistake 1960th Then Macheiramphus became the common generic name. Dean Amadon rejected Bonaparte's name by pointing out that it was a nomen oblitum , i.e. a forgotten name that should not be revived for the sake of nomenclature. However, since the use of Bonaparte's name increased from the 1960s and also had priority, Amadon could not prevail.
External system
As the only member of the genus Macheiramphus , the position of the bat hair within the hawk-like species was disputed for a long time, as its physique clearly differs from other genera. In the classical systematics Peters ' of 1931 and Amadon and Bulls of 1988 he was, together with the winged Kite ( Elanus ) and the Schwalbenschwanzaar ( Chelictinia riocourii ) in the sub-family of winged Kite provided (Elaninae). Knochenmorphologische investigations and studies of the mitochondrial and nuclear DNA , however, revealed that the bat hawk the Schwestertaxon the Harpyie ( Harpia harpyja ) of Papua Eagle ( Harpyopsis novaeguinae ) and the Würgadlers ( Morphnus guianensis group) and thus is in the group of basal Harpienverwandten. The family tree shown here follows a DNA analysis of the nuclear exon RAG-1 from 2007.
|
|
|
||||||||||||||||||
|
|
Internal system
A total of three subspecies are recognized for the bat pair:
- M. alcinus alcinus Bonaparte, 1850 : The nominate form is native to the Malay Peninsula, Sumatra, Sulawesi and Borneo.
- M. alcinus papuans ( Mayr ), 1940 : The New Guinean subspecies native to the Owen Stanley Mountains is smaller than the nominate form. It shows a white neck band and a whiter underside. Your forelock is less pronounced.
- M. alcinus anderssoni ( Gurney ), 1866 : With a size difference of 13%, the sub-Saharan subspecies shows a significantly stronger sexual dimorphism than the other two subspecies. She is smaller than M. a. alcinus and M. a. papuans . Tail feathers and hand wings are banded dark, the white coloring of the underside varies greatly.
Predators and causes of mortality

Little is known about the bat's predators. Attacks on adult birds have so far not been observed - apart from merely hating birds such as spotted uhus ( Bubo africanus ). However, corvids , especially shield ravens ( Corvus albus ) and vulture ravens ( Corvus albicollis ), often appear as nest robbers in southern Africa. They eat eggs and nestlings and are correspondingly often and aggressively hated by the bat pair. In addition, collisions with power lines, which are even more difficult to see at night than during the day, pose a risk to flightable bat hairs. Nothing is known about impairment caused by DDT , although bats are sensitive to this pesticide.
Existence and endangerment
The population of the bat pair is difficult to estimate due to its large distribution area. The fact that it is only active when it gets dark and retreats during the day makes an adequate estimate even more difficult. James Ferguson-Lees and David Christie assume with a hypothetical density of a breeding pair on 2000 km² from an at least five-digit number of individuals worldwide.
Since the bat pair does not depend on a specific type of habitat, it is in all probability not endangered. The IUCN has listed it as a least concern (not at risk) since 2004 ; before that it was already listed under lower risk (low risk) with a tendency towards least concern . As long as a sufficient stock of trees remains and the supply of specific food - bats, sailors and other nocturnal flying animals - does not decrease, James Ferguson-Lees and David Christie believe that the bat pair is not endangered. In addition, a critical population decline appears less likely because the bat pair is not dependent on a specific bat species. Locally, however, the increasing raven populations pose a threat to the resident bat population.
Cultural meaning

In Indonesia, the swallow's nest collectors paid shooting premiums for shot bat hairs, as this had a reputation for decimating stocks of black and white- nest salangans and thus harming the profitable business with their nests. Among the Penan and Punan on Borneo , the bat pair is considered an omentier that has not traditionally been hunted. They are trying to predict the future from its flight.
literature
- RK Brooke, PA Clancey: The authorship of the generic and specific names of the Bat Hawk . In: Bulletin of the British Ornithologists' Club 101, No. 4/1981. Pp. 371-372.
- Leslie Brown, Emil K. Urban , Kenneth B. Newman: The Birds of Africa. Volume 1 . Academic Press, 1988, ISBN 0-12-137301-0 , pp. 301-302.
- James Paul Chapin : Birds of the Belgian Congo . In: Bulletin of the American Museum of Natural History 65, 1932. pp. 545-551.
- John Barnard Dunning: CRC Handbook of Avian Body Masses . CRC Press, 2008. ISBN 1-4200-6444-4 , p. 49.
- James Ferguson-Lees, David A. Christie : Raptors of the World . Houghton Mifflin Harcourt, 2001, ISBN 0-618-12762-3 , pp. 94-95, pp. 350-352.
- MB Fenton, DHM Cumming, DJ Oxley: Prey of Bat Hawks and Availability of Bats . In: The Condor 79 (4), 1979. pp. 495-497.
- T. Harris, A. Kemp, J. Dunning: Nesting behavior of a pair of Bat Hawks Macheiramphus alcinus in South Africa, recorded by time-lapse video images . In: RD Chancellor, B.-U. Meyburg (Ed.): Raptors at risk . World Working Group on Birds of Prey, Berlin, and Hancock House, Blaine, WA 2000. ISBN 0-88839-478-0 .
- R. Hartley, K. Hustler: A less than-annual breeding cycle in a pair of African Bat Hawks Machaeramphus alcinus . In: Ibis 135, 1993. pp. 456-458.
- RR Hartley: Notes on the breeding biology and productivity of a pair of Bat Hawks in Mutare . In: The Honeyguide 41, 1995. pp. 6-17.
- Ron R. Hartley: Machaeramphus alcinus Bat Hawk. In: GH Verdoorn, Keith L. Bildstein, S. Ellis (Eds.): Selected African Falconiformes conservation assessment and management plan. IUCN / SSC Conservation Breeding Specialist Group, Apple Valley, MN 2000. pp. 64-65. (Online as PDF )
- M. Kemp, A. Kemp: Sasol Birds of Prey of Africa and Its Islands . Struik, 2006. ISBN 1-77007-369-8 , p. 198.
- Ernst Mayr : Birds collected during the Whitney South Sea Expedition. XLIII. Notes on New Guinea Birds VII . In: American Museum Novitates 1091, New York, November 15, 1940. (Online as PDF )
- Kenneth Newman: Newman's Birds of Southern Africa . Struik, 2006. ISBN 1-86872-735-1 , p. 204.
- Austin Roberts (Ed.): Roberts birds of Southern Africa . Voelcker Bird Book Fund, Cape Town 2005. ISBN 0-620-34053-3 , pp. 477-478.
- Craig Robson: Field Guide to the Birds of South East Asia . New Holland Publishers, 2009. p. 334.
- David R. Wells: The Birds of the Thai-Malay Peninsula. Volume I: Non-Passerines. Academic Press, 1999. ISBN 0-12-742961-1 .
Web links
- The bat pair on www.globalraptors.org
- Literature on the bat pair on the Global Raptor Information Network
Individual evidence
- ↑ a b c d e f g Ferguson-Lees & Christie 2001 , pp. 350–351
- ↑ a b Chapin 1932 , p. 547.
- ^ Kenneth Newman: Newman's Birds of Southern Africa . Struik, 2006, ISBN 1-86872-735-1 , p. 204.
- ↑ a b c d e f g h i Ferguson-Lees & Christies 2001 , p. 351.
- ^ Kemp & Kemp 2006 , p. 198.
- ↑ Species account: Macheiramphus alcinus . Global Raptors Information network, www.globalraptors.org, September 11, 2010. Retrieved February 25, 2011.
- ↑ a b Mayr 1940 , p. 1.
- ↑ a b c d e f g h i Roberts 2005 , p. 477.
- ↑ Ferguson-Lees & Christie 2001, p. 350.
- ↑ a b Hartley 1995, p. 6.
- ↑ a b Wells 1999 , p. 129.
- ↑ a b Harris et al. 2000 , p. 61.
- ↑ Fenton et al. 1979 , pp. 495-497.
- ↑ a b Hartley 1995, p. 13.
- ↑ Ferguson-Lees, Christie 2001, pp. 351-352.
- ↑ a b c d Ferguson-Lees, Christies 2001, p. 351.
- ↑ Hartley & Hustler 1993, pp. 456-458.
- ↑ Hartley 1995 , p. 15.
- ↑ Chapin 1936, pp. 550-551.
- ↑ Hartley & Hustler 1993 , p. 456.
- ↑ Brown et al. 1988 , p. 302.
- ↑ a b Hartley 1995, p. 16.
- ↑ Hartley & Hustler 1993, p. 457.
- ↑ Brooke & Clancey 1981, pp. 371-372.
- ↑ Carole S. Griffiths et al .: Phylogeny, diversity, and classification of the Accipitridae based on DNA sequences of the RAG-1 exon . In: Journal of Avian Biology 38/2007, pp. 587-602.
- ↑ Ferguson-Lees, Christies 2001, p. 352.
- ↑ Hartley 1995, pp. 13-16.
- ↑ Hartley 2000 , pp. 64-65.
- ↑ Macheiramphus alcinus in the IUCN Red List of Threatened Species 2011.2. Posted by: Birdlife International, 2009. Retrieved November 14, 2011.
- ↑ Clive Roots: Nocturnal Animals. Greenwood Publishing Group, 2006. ISBN 0-313-33546-X , p. 68.
- ↑ Peter Sercombe, Bernard Sellato: Beyond the Green Myth: Borneo's Hunter-Gatherers in the Twenty-First Century . NIAS Press, 2008. ISBN 87-7694-018-7 , p. 183.