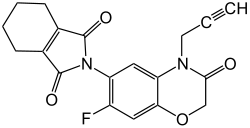
Flumioxazin
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Flumioxazin | |||||||||||||||
| other names |
N - (7-fluoro-3,4-dihydro-3-oxo-4-prop-2-ynyl-2 H -1,4-benzoxazin-6-yl) cyclohex-1-en-1,2-dicarboximide |
|||||||||||||||
| Molecular formula | C 19 H 15 FN 2 O 4 | |||||||||||||||
| Brief description |
yellow-brown powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 354.33 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.51 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
201-204 ° C ° C |
|||||||||||||||
| Vapor pressure |
3.2 mPa (22 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Flumioxazin is a chemical compound from the group of the N -phenylphthalimide derivatives and N -phenylimides . It is used as a herbicide .
Extraction and presentation
Flumioxazin can be obtained by a multi-stage reaction starting from 3-fluorophenol with chloroacetic acid , nitric acid , ethanol , propargyl chloride and 3,4,5,6-tetrahydrophthalic anhydride .
properties
Flumioxazin is a yellow to slightly brown solid that is practically insoluble in water.
use
The herbicide Flumioxazin was developed by Sumitomo Chemical and is approved in Canada for use in fruit, vegetable and ornamental crops as well as for weed control in industrial plants against ragweed , dandelion and nightshade plants . It was introduced in the US in 2001 for use on peanuts and soybeans . In 2011, almost 500 tons were used there.
The mode of action is based on the inhibition of protoporphyrinogen oxidase (PPO), an enzyme that is important for the synthesis of chlorophyll . Flumioxazin has a half-life of around 2 to 10 hours in water and 12 to 17 days in soil.
In Germany, Austria and Switzerland, plant protection products (e.g. Nozomi, Sumimax, Vorox F) that contain flumioxazin as an active ingredient are approved.
Individual evidence
- ↑ a b c d e f data sheet Flumioxazin PESTANAL®, analytical standard at Sigma-Aldrich , accessed on July 14, 2017 ( PDF ).
- ↑ a b c d Directorate-General for Health and Food Safety of the European Commission: Entry on Flumioxazin in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on July 14, 2017.
- ↑ Flumioxazin data sheet . Federal Office for Consumer Protection and Food Safety , accessed on July 14, 2017 (PDF; 19 kB).
- ↑ a b c Entry on Flumioxazin in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ Entry on N- (7-fluoro-3,4-dihydro-3-oxo-4-prop-2-ynyl-2H-1,4-benzoxazin-6-yl) cyclohex-1-ene-1,2- dicarboxamide in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Thomas A. Unger: Pesticide synthesis handbook . 1996, ISBN 978-0-8155-1401-5 ( page 428 in the Google book search).
- ↑ Engageagro: Flumioxazin Herbicide ( Memento from January 11, 2015 in the Internet Archive ) (PDF; 113 kB).
- ↑ Gad Loeb Stone, George Thottappilly: The Sweet Potato . 2009, ISBN 978-1-4020-9474-3 ( page 312 in the Google book search).
- ↑ JH Daugrois, JW Hoy, JL Griffin: Protoporphyrinogen Oxidase Inhibitor Herbicide Effects on Pythium Root Rot of Sugarcane, Pythium Species, and the Soil Microbial Community . In: Phytopathology . tape 95 , no. 3 , 2005, ISSN 0031-949X , p. 220–226 , doi : 10.1094 / PHYTO-95-0220 (free full text).
- ↑ Entry on Flumioxazin. In: Römpp Online . Georg Thieme Verlag, accessed on July 14, 2017.
- ^ Q. Ashton Acton (Ed.): Advances in Ecology Environment and Conservation Research and Application: 2012 Edition . ScholarlyEditions, Atlanta 2012, ISBN 978-1-4649-9111-0 ( limited preview in Google Book Search).
- ^ Thomas J. Monaco, Stephen C. Weller, Floyd M. Ashton: Weed Science: Principles and Practices . John Wiley & Sons, New York 2002, ISBN 978-0-471-37051-2 , pp. 248 ( limited preview in Google Book search).


