Feed water
When feed water is water in one referred to the feedwater tank is maintained and continuously to a steam generator is supplied. Large quantities of feed water are required in steam power plants . The steam boiler generates steam that is used for heating, for procedural processes or to drive a steam turbine or steam engine. The feed water is treated in such a way that components of the water that are harmful to the operation of the boiler have been removed or converted into substances that have no negative effect on the boiler operation.
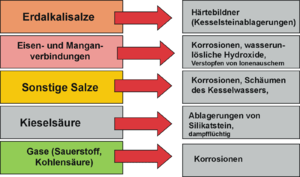
Harmful constituents of the water used are salts of the alkaline earths , which precipitate on the heating surfaces at higher temperatures, form an insulating layer and thus hinder the heat transfer . This leads to overheating with the consequence of thermal stress cracks. In addition, the boiler scale can render safety-relevant equipment inoperative through deposits. The gases O 2 and CO 2 dissolved in the water lead to corrosion . With once-through boilers, all of the feed water is evaporated. With this type of boiler, all dissolved ingredients in the raw water must therefore be removed from the feed water. Therefore only deionized water (= pure water without ingredients) may be used.
Depending on the use of the steam, more or less steam can be used as condensate again as feed water. In a steam power plant, the losses due to blowdown and thermal degassing must be compensated for with additional water. In process engineering systems, the steam is sometimes used for direct heating so that no condensate is available for further use.
The feed water consists of the re-used condensate and treated make-up water.
condensate
The condensate is collected in the condensate container and fed to the feed water container by means of condensate pumps. The quality of the reused condensate may have to be checked if there is an ingress of foreign matter - e. B. on a leaky heat exchanger - is possible.
When driving steam engines, the condensate is loaded with oil. If larger quantities of oil get into the boiler water, there is a risk of overheating of the heating surfaces , since film boiling starts at lower heat flux densities. The oil has to be separated mechanically by floating and the residues have to be adsorbed by an activated carbon filter.
Depending on the substances that may penetrate, automatic turbidity monitoring or conductivity measurement can be used, which controls a 3/2-way valve and feeds the condensate to the expansion valve. This facility is compulsory for operations without constant supervision ( TRD 604). In the case of high-pressure boilers (operating pressure> 64 bar), the condensates must be processed, especially with through-flow boilers. Undissolved components such as iron oxides, which are detached from the material of the pipes through corrosion or erosion, and traces of salt that have entered the steam / condensate system from the cooling water in the condenser or in various heat exchangers must be removed. Typical systems for condensate processing are candle filters (for removing undissolved particles) and mixed-bed filters (for removing dissolved salts).
advancement
One or more feed water pumps are located between the feed water tank and the inlet of the feed water into the steam generator .
treatment
Thermal degassing
As a rule, the feed water is degassed in a degasser that is arranged on the feed water tank. This largely removes the harmful gases oxygen and carbon dioxide. The physical fact that the solubility of gases in liquids decreases with increasing temperature is used for degassing. Further information on the physical process of degassing can be found under degassing (steam and hot water technology) .
The degassing of the feed water is mainly used at overpressure (p = 0.1–0.4 bar / g) and corresponding saturated steam temperatures of 102–108 ° C. The feed water tank is heated by a steam lance in the water space to mix the liquid and by adding steam in the gas space. A degasser dome is flanged onto the top of the feed water tank. The cold make-up water and the condensate are directed to the upper distributor plate and run over trickle plates into the feed water tank. Steam flows in countercurrent to the head of the degasser and heats the water introduced. The bound gases are released and rise with the steam. The rising vapors escape at the head of the degasser and are referred to as sweeping steam . A vapor condenser is used in larger systems to utilize the enthalpy of the vapors. The treated make-up water is preheated through the condensation of the water vapor in the vapors.
The allowable pressure of the feed water tank can be safeguarded by a safety valve or a siphon. A siphon can only be used with degassers at operating pressures of up to around 1.5 bar, as otherwise the pipe loop (necessary for the required static water column as counterpressure) will be too high. However, the use of a siphon has the advantage that if the flow control fails, the feed water tank cannot overfill.
In addition to the degassing described above at a pressure of> 1.0 bar, there are vacuum degassers. In terms of structure and mode of operation, these are largely comparable to pressure deaerators. The operating pressure is below 1.0 bar, depending on the operating temperature. The vacuum is generated by vacuum pumps (mainly water ring pumps and / or steam jets). For condensates to be degassed with a raw condensate temperature of approx. 35-100 ° C, so-called expansion degassers can also be used, the construction of which is largely comparable to normal vacuum degassers.
Chemical degassing
In addition to the above-mentioned physical degassing, catalytic degassing is also possible for the make-up water. Here, a resin loaded with a heavy metal from the platinum group is used as a catalyst. Hydrogen or hydrazine is used as a reducing agent. The removal of oxygen takes place in a filter, which is comparable in construction to an ion exchange filter. The operating temperature for this process is typically 10-30 ° C.
Low oxygen contents in condensates from boiler systems in industry and power plants are also chemically bound. The reducing agents are added after the condenser and after the degasser to remove residual oxygen. This treatment protects system parts made of iron from corrosion by oxygen. By dosing after the degasser, small residual contents of approx. 5–10 ppb oxygen can be chemically reduced even further.
The following chemicals are suitable and commonly used for this type of oxygen degassing:
- Sodium sulfite
- Diethylhydroxylamine (DEHA)
- Hydrazine
- Morpholine
- 2-butanone oxime as methyl ethyl referred
- Ascorbic acid as ammonium ascorbate
- Carbodihydrazide (also known as carbohydrazide or diaminourea )
With the exception of sodium sulfite (Na 2 SO 3 ), these chemicals are volatile and can be used in systems with forced-flow boilers . The use of sodium sulfite, on the other hand, is limited to systems with circulation boilers and hot water circuits.
Hydrazine was predominantly used until the 1980s. Since only nitrogen and water are formed when hydrazine reacts with oxygen, it is an optimal chemical for oxygen removal. However, since hydrazine is one of the carcinogenic chemicals according to the Hazardous Substances Ordinance, the less dangerous other agents are increasingly being used. The disadvantage of these substitutes, however, is that they form organic breakdown and breakdown products such as organic acids, carbon dioxide, aldehydes or ketones. The COD content in the water-steam cycle is increased and the quality deteriorates.
Cold degassing
In addition to classic thermal degassing and chemical degassing, cold degassing also offers the possibility of removing dissolved oxygen from the feed water. This method is particularly suitable for high-speed steam generator systems because, especially with little or no condensate being returned, the soft water does not have to be heated up for degassing, but rather, already low in oxygen, is introduced into the feed water tank, which means that expensive oxygen binders can almost be dispensed with . Since the feed water in high-speed steam generators is usually not heated above 95 ° C and proper mixing, which would be necessary to give chemical oxygen binders a guaranteed effect, is very rarely used, cold degassing is the only effective method here the values needed to come.
Further treatment of the feed water
Feed water treatment with exchange softening must be supplemented by removing or binding the oxygen and using water conditioning agents that bind the residual hardness builders. Usually the gases (oxygen, carbon dioxide) are removed by thermal degassing at the feed water tank. By adding sodium sulfite, the residual oxygen is chemically converted into sodium sulfate. Hydrazine can also be used to chemically bind oxygen. By adding trisodium phosphate, the hardness components are set and excreted as sludge. The pH value is increased by adding caustic soda or ammonia . The dosage of salts such as trisodium phosphate and caustic soda is only permitted for drum boilers. For once-through boilers, only chemicals that are volatile can be used, as salts would crystallize out on the heating surfaces in the area of residual water evaporation.
In order to simplify water treatment, these water conditioning substances are usually supplied as a ready-made mixture. These agents are filled into plastic containers and pumped into the feed water container or into the make-up water by means of membrane pumps. In order to obtain an even dosage, the pump is activated at the same time as the feed pump. The delivery rate of the dosing pump is set so that an excess of the components of the treatment agent can be detected in the boiler water.
Make-up water
The additional water used must be treated in order to prevent the ingredients from having a harmful effect on the boiler.
Depending on the application and in particular depending on the operating pressure of the boiler, a distinction is made between the following treatment stages:
- salty feed water,
- low-salt feed water,
- salt-free feed water.
Insoluble constituents must first be removed from the make-up water . Coarse components can be removed using rakes or in settling basins. The finer components are removed by filtration or flocculation .
| Hardness range | designation | Total hardness |
|---|---|---|
| 1 | soft | 0 to 1.3 mmol / l |
| 2 | medium hard | 1.4 to 2.5 mmol / l |
| 3 | hard | 2.6 to 3.8 mmol / l |
| 4th | very hard | 2.6 to 3.8 mmol / l |
In well water and surface water there are often harmful amounts of iron and manganese compounds , which are converted into sparingly soluble oxides through oxidation and form deposits. Dissolved iron and manganese are removed when the water is aerated and the resulting water-insoluble hydroxides are separated in a gravel filter . Oxygen and free carbon dioxide dissolved in the raw water cause corrosion, especially in steel system components, and must be removed.
Internal feed water treatment
see main article: Internal feed water treatment
Internal feed water treatment means treatment of the water in the boiler. Except for the previously separated solids, all components of the water used get into the boiler. The oxygen must be bound by sodium sulphite or other suitable means and carbon dioxide by caustic soda. Phosphates are added to the feed water, which react with the hardness builders and form calcium or magnesium phosphate, which collects as sludge on the boiler bottom. The boiler must therefore often be drained. This form of water treatment is only permitted as the only measure for shell boilers without smoke tubes (flame tube boilers) and steam locomotive boilers and is therefore of little importance.
In today's boiler systems, hardness components and dissolved oxygen are largely removed from the feed water before it is fed to the boiler (see also external feed water treatment). The remaining damaging water components are converted by water conditioning agents, which in particular contain sodium sulfite, phosphates and basic components to raise the pH value.
External feed water treatment
External feed water treatment means that the unwanted constituents of the water used are physically or chemically removed before being fed into the steam boiler or converted into compounds that do not cause any damage to the boiler or the steam consumers.
Ion exchangers are used in modern water treatment plants. These consist of containers that are filled with spherical synthetic resins with a grain diameter of 0.3–1.5 mm. The exchangers work regeneratively; this means that the ion exchanger is reset to the original, unloaded state after regeneration. The regeneration takes place through treatment with acids, alkalis or salts. Double exchangers are often used so that the other can be used when regenerating one exchanger. Before treatment with chemical solutions, the exchangers are often backwashed. This backwashing removes any impurities that have been filtered off and resin debris. Furthermore, the resin bed is loosened, which prevents channel formation during regeneration.
Exchange softening
For small and medium-sized steam boiler systems up to medium steam pressures (approx. 32 bar), only exchange softening with downstream chemical conditioning and degassing is carried out. This softening takes place in a cation exchanger, as the positive alkaline earth ions are exchanged here. The exchanger is regenerated with inexpensive table salt . The hardness components calcium and magnesium , which were attached to the ion exchange resin during the loading, are replaced by the sodium ion of the regeneration agent common salt. 8-10% saline solution is used for regeneration . This must take place in excess (180–250% of the equilibrium amount ) in order to establish an excess of sodium ions that deviates from the equilibrium between calcium and sodium ions in the exchanger mass. There is thus a chemical compulsion to bind the calcium ions in the resin and release the sodium ions. There is a chemical compulsion in the exchanger to reduce the free enthalpy through the ion exchange.
A residual alkaline earth content of <0.01 m mol / l is achieved. With exchange softening, the hardness builders are replaced and thus the salt content of this treated water is not reduced. The boiler exposed to the water must therefore be salted down accordingly, so that a dangerous foaming of the boiler water is avoided. The use of exchange softening when using water with a very high total hardness and a high content of sodium salts is problematic. The softening by means of ion exchange does not reduce the anions - hydrogen carbonates , chlorides and sulfates . The feed water then contains high levels of these sodium salts and has a corrosive effect on the ferrous materials of the boiler, the pipes and the heat exchangers.
Partial desalination
Reverse osmosis
A method of partial desalination that has been used recently is reverse osmosis , as here, compared to ion exchangers, regeneration can be dispensed with and continuous plant operation is possible. The prerequisite for use is an upstream softening of the water. The water must be clear and free from insoluble foreign matter, in particular free from organic impurities, in order to avoid blocking of the membranes. The principle of reverse osmosis is based on the fact that the diffusion resistance of the pores of the membranes used is significantly lower for the smaller water molecules than the resistance of the larger ions dissolved in the water. The water is fed into the membrane-equipped modules at a higher pressure. The level of pressure required depends on the salinity of the raw water and the type of membrane used. The pressure in fresh water is usually around 20 ± 5 bar. With sea water the pressure is approx. 50 ± 20 bar. Water and a proportion of the smaller salt ions in particular diffuse through the membrane and form the permeate , which is available as partially desalinated water. The permeate content of the water used is 70–90%. The rest is made up of the salt-rich concentrate , which has not diffused through the membrane. This part is discarded.
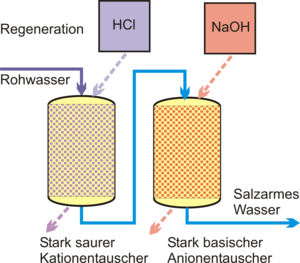
Ion exchange
In order to achieve desalination of the feed water, the make-up water is passed through a strongly acidic cation exchanger in the hydrogen form and then through a strongly basic anion exchanger . The cations are bound in the cation exchanger and replaced by the hydrogen ions (H + ) deposited in the resin . The anions are therefore present as free acids behind the exchanger. In the basic exchanger in the hydroxyl form, the anions are bound and replaced by a hydroxyl ion (OH - ). The strongly basic anion exchanger is also able to bind carbonic and silicic acid.
Natural waters that contain colloidal silicon dioxide (SiO 2 ) can cause problems when they are processed into boiler feed water. This has to be taken into account, especially with full demineralisation with ion exchangers. Ion exchange resins do not remove non-ionic compounds, or only remove them slightly, from water. When treating such water, an additional flocculation stage to remove these colloids must therefore be connected upstream of the ion exchange.
The cation exchanger is regenerated by hydrochloric or sulfuric acid when it is exhausted ; Caustic soda is used for the anion exchanger .
With this arrangement, all dissociated substances (salts, acids, alkalis) can be removed from the water. In the case of water with a high proportion of hydrogen carbonates , a CO 2 trickle is included between the two exchangers in order to relieve the anion exchanger. The achievable residual conductivity of a cation-anion exchanger arrangement is <10 μS / cm.
Full demineralization
A complete desalination of the water with a conductivity below 0.2 μS / cm is achieved by the arrangement with CO 2 trickle described in ion exchange if a mixed-bed filter is also installed behind the anion exchanger. Cation and anion exchangers are combined in the filter. In order to optimize the demineralization and to reduce the amount of regeneration agent, weakly acidic and weakly basic anion exchangers are added upstream of the strongly acidic and basic exchangers in the preparation steps. The fully demineralized water is also known as deionized water . Modern full desalination plants mainly work according to the countercurrent principle . The directions of flow are opposite during loading and regeneration in the resin bed. When using multi-chamber containers, weakly and strongly acidic cation resins or anion resins can be combined. With only a small excess of chemicals - often less than 105% of the theoretical value - an already largely ion-free deionized material without a mixed bed filter with a conductivity of <1.0 µS / cm is generated.
Requirements for the feed water quality
Trouble-free long-term operation for steam boilers and steam power plants can only be achieved for the system components through which water and steam flow ( piping systems , boiler tubes , heat exchangers , containers , turbines , pumps and condensers ) if corrosion on all system parts is prevented as far as possible. In addition to the correct selection and combination of suitable materials, optimal feed and boiler water quality is a prerequisite for this. In general, the following must be observed for the feed water:
- Materials made of iron and copper are only resistant to water and steam if there are dense and crack-free oxide layers at the boundary layer between medium and metal
- The particularly erosion-resistant protective layers made of magnetite are only formed at water temperatures from 200 ° C; the solubility of magnetite increases at pH values <9.4
- The corrosion rate of ferrous materials has a minimum at a pH value of> 9.1, the minimum corrosion rate for copper materials in the presence of ammonium is at a pH value of 8.5
- the distribution coefficient of the alkalizing agents, for example for ammonia solution, is different for the liquid and the vapor phase. In the vapor phase of circulation boilers, the ammonia content is significantly higher than in the water phase. In the condenser (condensate), therefore, locally significantly higher ammonia contents will occur. This can lead to severe corrosion on capacitors with brass tubing.
- For the lowest possible corrosion rates, the electrolyte (salt) content in the water and steam must be as low as possible, corresponding to a conductivity of ≤ 0.2 µS / cm. The conductivity caused by alkalizing agents is not taken into account.
- the content of dissolved free oxygen in the system should normally be <0.005 mg / l; In water with pH values of 7.0 to 9.0, the corrosion rate is lower in salt-free water (<0.2 µS / cm conductivity) but with oxygen contents of 0.15-0.30 mg / l
- The pH range selected during operation for the water and vapor phases in the system is of particular importance in practice.
The above conditions are partly contradicting one another. Therefore compromises are necessary for optimal conditions, which take into account the entire system with the different materials and temperature conditions. The following qualities for the feed water are common in Germany for high pressure systems and the operating phase:
- Neutral driving style
- Combined driving style
- Alkaline driving style
- High AVT driving style. (AVT = A ll V olatile T reatment)
The following table with basic data on the control strategies:
| Driving style | pH value / O 2 content | Dosage of | Corrosion rate | System note ° 1 |
|---|---|---|---|---|
| Neutral driving style |
|
|
|
only possible with electrolyte-free conditions with ≤ 0.20 µS / cm ° 2 in the overall system |
| Combined driving style |
|
|
|
used in systems with iron and brass-tubed condenser |
| Alkaline driving style |
|
|
|
mainly used in systems that are free from copper-containing materials |
| High AVT driving style |
|
|
Total Fe |
applied in the secondary systems of nuclear power plants with pressurized water reactors |
° 1 Note: In a study from the beginning of the 1990s in Germany, the following figures are given for the different driving styles:
- Neutral driving style: 17 systems
- Combined operation: 65 systems
- Alkaline operation: 150 systems
° 2 Note: conductivity measured directly; For modes of operation with ammonium hydroxide metering: after strong acidic cation exchange to remove the ammonium hydroxide before the measurement
Guidelines for boiler and feed water
The required quality of this water is specified in guidelines. The following guidelines in particular must be observed when operating the different boiler types and pressure levels:
- VGB guidelines for feed water, boiler water and steam quality for power plants / industrial power plants (VGB = VGB Power Tech )
- Technical rules for steam boilers -TRD- from the German Steam Boiler Committee (DDA) and the Association of Technical Monitoring Associations (VdTÜV). In TRD 611, for example, the data for group IV steam generators are given.
The data for large capacity water boilers are given in the following list:
Requirements for feed water for shell boilers according to EN 12953 part 10 (except injection water)
| parameter | unit | Feed water for steam boilers with solids content | Make-up water for hot water boiler | |
|---|---|---|---|---|
| operating pressure | bar | > 0.5 to 20 | > 20 | Total area |
| Appearance | Sure, free of suspended matter | |||
| pH value at 25 ° C | > 9.2 | > 9.2 | > 7 | |
| Total hardness (Ca + Mg) | mmol / l | <0.01 (PS <5 bar to 0.05) | <0.01 | <0.05 |
| iron | mg / l | <0.3 | <0.1 | <0.2 |
| copper | mg / l | <0.05 | <0.03 | <0.1 |
| Silica (SiO 2 ) | mg / l | not specified, only reference values relevant for boiler water | - | |
| oxygen | mg / l | <0.05 | <0.02 | - |
| Oil / fat | mg / l | <1 | <1 | <1 |
See also
literature
- WJF van der Wal: In: VGB power plant technology. Born in 1969, No. 3, pp. 296-299.
- Fritz Mayr: Boiler operating technology. 10th edition. Publishing house Dr. Ingo Resch, 2003, ISBN 3-930039-13-3 , pp. 394-397.
Individual evidence
- ↑ P. Thomas: Operating experience with catalytic reduction. In: VGB power plant technology. 61, 1981, No. 1, pp. 59-61.
- ↑ L. Braunstein, K. Hochmüller, K. Sprengler: The determination of colloidal silica in water. In: VGB power plant technology. Vol. 62, Sept. 1982, Issue 9, p. 789.
- ↑ A. Bursik et al. a .: In: VGB power plant technology. Vol. 60, 1980, No. 6, p. 487.
- ^ A b A. Bursik, G. Resch: In: VGB Kraftwerktechnik. Vol. 61, 1981, No. 4, p. 288.
- ↑ P .H. Efferts, W. Fichte, B. Szenker, G. Resch, F. Burgmann, E. Grünschläger, E. Beetz: Combined oxygen / ammonia conditioning of water-steam cycles. In: The machine damage. Vol. 51, 1978, issue 3, p. 116.
- ↑ R. Turyna, V. Svarc: contribution to the review of some equations of dissociation and partition coefficient of ammonia. In: VGB power plant technology. Vol. 62, 1982, issue 1, p. 459.
- ↑ A. Bursik: In: VGB power plant technology. Vol. 62, 1982, issue 1, p. 41.
- ↑ a b c A. Bursik: In: VGB power plant technology. Vol. 62, 1982, issue 1, p. 40.
- ↑ A. Dörr, S. Odar, P. Schub: In: VGB Kraftwerktechnik. Vol. 66, 1986, issue 11, p. 1059.
- ↑ A. Bursik, H. Kittel: In: VGB power plant technology. Vol. 72, 1992, issue 2, p. 166.