Medical technology
Medical technology , also called biomedical technology , is the application of engineering principles and rules in the field of medicine. It combines technical knowledge , especially problem solving and development, with the medical expertise of doctors , nurses and other professions to improve the diagnosis , therapy , nursing , rehabilitation and quality of life of sick or healthy individuals. In English, the terms (bio) medical engineering and (bio) medical technology are common.
European harmonization
European legislation has brought about a harmonization of national legislation, which is implemented autonomously in the individual member states of the European Union. Special features of the assignment to individual legal works are retained nationally. The naming of the national laws varies and can be assigned by reference to the European rules. The relevant guidelines and standards include the following and other guidelines and harmonized standards :
- Directive 90/385 / EEC on active implantable medical devices
- changed several times
- Directive 93/42 / EEC on medical devices
- modified by amending directive 2007/47 / EC
- Directive 98/79 / EC on in-vitro diagnostic medical devices
- (DIN) EN ISO 13485 - Management system for the design and manufacture of medical devices
- (DIN) EN 60601 (or VDE 0750) - complex of standards for medical electrical devices and systems
- (DIN) EN ISO 11607 on packaging for medical products
- (DIN) EN ISO 14644 on clean rooms and clean room areas
- (DIN) EN ISO 14698 on biocontamination controls of clean rooms and clean room areas
- (DIN) EN ISO 14971 - Manufacturer-side risk management for medical devices
- (DIN) EN ISO 15223-1 on medical device labels
As an area that is constantly growing with medical and technical progress, the task of medical technology consists of
- Research and Development (R&D), e.g. B. in the following areas:
Medical informatics , signal processing of physiological signals, biomechanics , biomaterials and biotechnology , system analysis , creation of 3D models, etc. Examples of specific applications are the production of biocompatible prostheses, medical therapy and diagnostic devices, such as B. EKG recorders and ultrasound devices , imaging diagnostics, such. B. Magnetic resonance imaging (MRI), and electroencephalography (EEG) and the manufacture of new drugs
- Preparation for supporting medical work
- Preparation for supporting nursing work
In the future, the European Medical Devices Regulation (MDR - (EU) 2017/745) and the In-Vitro Diagnostics Regulation (IVDR - (EU) 2017/746) will replace the existing medical device directives and IVD directives.
Legal framework
The German Medical Devices Act (MPG) , for example, can serve as a demarcation : Medical technology produces devices, products and technical processes that are medical devices . This definition ranges from simple bandages to large medical devices and complete systems. The German Medical Devices Act (MPG) implements the requirements of the European directives 90/385 / EEC for active implantable medical devices, 93/42 / EEC for medical devices and 98/79 / EC for in-vitro diagnostics at national level.
Economic classification
The manufacturers of medical technology are important for the national economies.
Are characteristic of medical technology
- a close interlinking of products and services
- extensive national, supranational and international standardization
- extensive ongoing research and development.
- Comprehensive government regulations to protect patients, payers and manufacturers
- diverse national features
- High prices corresponding to the accompanying expenditure and not solely determined by the use of materials
Areas of medical technology
Hospital technology

Hospital engineering ( English clinical engineering ) is a sub-area of medical engineering that deals with medical devices and medical products in hospitals. The tasks of an engineer in this area are to advise on the acquisition and management of medical devices and to supervise medical technicians in order to ensure that safety and legal requirements are met during their work. The engineers also act as consultants for all questions relating to the use of medical products. Engineers in this field work closely with hospital IT and medical physicists. If there is no corresponding training qualification , the MPG requires the qualification as a medical device advisor for such an activity .
A typical medical technology department deals with the repair and preventive maintenance of all medical devices, with the exception of devices that are still covered by a guarantee or a maintenance contract. All newly acquired devices are first checked for compliance with safety regulations before they are used. These are e.g. B. Checking the leakage current, checking the collision-free movement sequences or the risk of crushing, function of emergency stop buttons and, if necessary, radiation or image quality measurements. In most devices, not all parameters of a function are tested, but so-called equivalence classes of parameters are formed in order to make testing cheaper. Nevertheless, it is ensured that the test is carried out correctly and conscientiously.
Many medical devices have to be sterilized before they can be used. This is a particular problem as most sterilization processes can damage materials and equipment.
Most medical devices are either inherently safe or have devices and systems ( watchdogs ) that can detect a failure and turn the product into an unusable and therefore safe state. A typical requirement is first failure safety. This means that not a single first error can lead to an unsafe use of the device during its service life.
Medical equipment
The purpose of a medical device:
- Detecting, preventing, monitoring, treating or alleviating disease;
- Detecting, monitoring, treating, alleviating or compensating for an injury or disability;
- Investigation, replacement or modification of the anatomical structure or a physiological process;
- Conception control
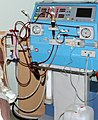
Some examples are pacemakers , infusion pumps , heart-lung machines , dialysis machines , artificial organs , visual aids , cochlear implants , prostheses of all kinds and dental implants .
Medical devices are divided into different classes, in which active and passive medical devices are differentiated and there is a further subdivision into four risk classes I, IIa, IIb and III.
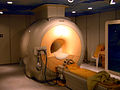
Imaging diagnostics
Diagnostic imaging devices are among the most complex medical products in any hospital. Depending on the tissue to be displayed, various methods with or without a contrast agent are used to obtain structural (morphological) and / or functional (physiological) information. Examples are:
Without ionizing radiation :
With ionizing rays :
Tissue engineering
One of the goals in tissue engineering is to create artificial organs for patients who need an organ transplant. Medical technology engineers are currently researching methods of growing such organs. Examples of successfully transplanted organs are bladders. Other artificial organs using both biological and synthetic components are also an area of research, e.g. B. the production of liver replacement devices that use liver cells that have been produced in an artificial bioreactor .
Medical informatics
According to the intentions of the legislature, information technology facilities have recently also been included in medical technology if their functions are decisive for administrations and applications that can endanger the patient.
Medical technology industry
Germany is the world's third largest manufacturer of medical technology after the United States and Japan . In 2012, the global medical technology market was estimated to be $ 331 billion. The USA accounts for around 40% of the world market and Europe for around 30%.
The 1177 German medical technology manufacturers achieved total sales of 24.1 billion euros in 2012 with around 119,000 employees. The 392 companies with 50 employees and more accounted for almost 95,000 employees and a turnover of 22.3 billion euros (2013: 418 companies, 98,000 employees, 22.8 billion euros in turnover). There are also around 11,460 small and trading companies and sales branches with a further 70,000 employees. A total of almost 12,640 medical technology companies with more than 189,000 employees are active on the German market. Another 35,000 people are employed in the retail trade with medical and orthopedic items. The industry is largely characterized by medium-sized companies: 93% of the 1,177 companies employ fewer than 250 people. The companies generate around 2/3 of their sales (68%) abroad.
In 2015, the roughly 1230 German medical technology manufacturers generated total sales of € 27.6 billion and an increase in sales of around 9%. Domestic sales in 2015 as a whole increased by 10 percent and reached a value of almost 10 billion euros. For international business there is an increase of almost 8 percent. The foreign turnover is 17.6 billion euros. This results in an export quota of 64 percent.
The associations BVMed, SPECTARIS and the Association for Electromedical Technology of the ZVEI are important representatives of the industry in Germany.
Statutory Regulations
Legal regulations must always remain in the back of the mind of a medical technology engineer. In order to comply with safety regulations, the manufacturers of most medical devices must demonstrate that they are managed, designed, manufactured, tested, supplied and used in accordance with the conditions and approvals. The purpose of this measure is to ensure the quality and safety of diagnosis and therapy by reducing the likelihood of accidentally missing important steps.
In Europe, the decision as to whether a medical device is used is made by the prescribing doctor, and the existing legal regulations are intended to ensure that these are both safe and effective, i.e. help more than harm and function accordingly. For this reason, medical devices are certified by notified bodies . Technical committees of leading scientists write recommendations that are written after discussion and the inclusion of public comments in EU directives (or regulations) . These guidelines vary depending on the product and contain regulations for the development and testing of the safety and effectiveness of a medical device. In the EU, medical devices may only be placed on the market or put into operation if they are provided with a CE mark . The national legal regulations for medical devices are in medical law, both in Germany and in Austria in the national Medical Device Act additionally (MPG), as well as in Germany in the Medical Device Directive (MPBetreibV) and the Law on Metrology and Verification find are.
Some medical devices have to be subjected to a safety control (STK) and possibly a metrological control (MTK) at regular intervals ; this is usually done by the medical technician .
education
Several job profiles have emerged for planning, development, maintenance and sales of medical-technical products. In Germany, these include, among other things, the training occupation as medical technician and the university degree Dipl.-Ing. for medical technology, Bachelor of Science and Master of Science in medical technology.
Medical technician apprenticeship
Medical technicians have completed qualified vocational training in the metal or electrical sector and have also received additional qualifications. This can be done either through several years of practical work experience with medical technology devices or through a two-year further education at a technical school with the qualification as a "state-certified technician, (specialization) medical technology".
Medical engineering degree
In Germany, the previous diploma courses are being converted to a Bachelor / Master’s program, and with the switch to Bachelor / Master’s programs, universities are increasingly offering the medical technology course. The study of medical technology includes well-founded basics in both engineering and biological sciences, e.g. B. Physiology , and is therefore usually completed with a Bachelor or Master of Science. The number of universities that offer this course is growing rapidly, as the research area is constantly growing. This is particularly a response to the increasing need for interdisciplinary training and research as well as the enormous innovative strength in this research area.
It is also widespread at universities that specialization in medical technology is offered as part of engineering or physics courses.
The German Society for Biomedical Technology ( DGBMT ), a specialist society in the Association of Electrical, Electronics and Information Technology , divides the topics for the study of medical technology as follows:
- Medical physics
- Medical informatics
- biomedical technology
-
Clinical engineering
- Biomechanics
- Biomaterials & Artificial Organs
- Molecular Biology , Cellular & Tissue Engineering
- Hygiene technology
- Laboratory & analysis technology
- Approval of medical devices
-
Health Economics & Ethics
- Physiology & anatomy
- Medical terminology
The DGBMT presents the topics in a circle and assigns them more or less precisely to the individual generic terms.
Since 2014, the Medical Technology Conference (KOMET) has been held at least once a year as a federal student council meeting for medical technology. Around 25 universities and technical colleges had participated in the conference at least once by 2018.
In the USA , it is often a Masters or PhD program in which students from a wide variety of subjects in engineering or natural sciences deepen their knowledge. But it is also growing rapidly as a Bachelor program. Often it is also used as a bachelor's degree before starting medical studies, as it teaches students the fundamentals from a wide range.
research
Medical technology research can be basic scientific and technical research that promises possible applicability in medicine. It can also be dedicated medical-technical basic research, preliminary research with a clear product reference or technical product development.
The corporate landscape is extremely heterogeneous (between 1 and 10,000 employees, R&D budgets between 0 and 50% of sales). Overall, medical technology is a sub-area with an above-average proportion of research. The industry average cost share for research and development is approx. 9.5% of sales; 14.7% of the employees are engaged in research (2001). German companies hold the second highest number of relevant patents (after US companies) and generate 50% of their sales with products that have been on the market for less than two years.
Since the content of industrial research is secret, official statistics relate primarily to the public sector in universities and institutes ( Fraunhofer Society , Max Planck Society, etc.). The u. G. Inventory of the Federal Ministry for Research and Technology (see web links) covers over 1100 public medical technology research projects in Germany. These focus on information technology, imaging processes, biomaterials, cell and tissue technology. The BMFT budget for this is around € 35 million per year.
In the international literature, "imaging procedures" (MRT, X-ray, endoscopy) are by far the most important medical technology research area. The importance of the individual countries follows the economic situation. Germany has a share of approx. 15%, it is particularly leading (with 60% of all publications) in the areas of “multislice CT” and (with 40%) “ magnetic resonance imaging ”.
Medical technology costs
The effective direct expenditure for medical technology is estimated at 5% of the total health expenditure, according to the Association of Swiss Medical Technology Fasmed at less than 5% (medical technology in Switzerland). Pammolli et al. give 4.5% for Switzerland (2002). According to the European umbrella association of medical device companies Eucomed, the figure is 6.4% in Europe, 4.6% in Switzerland (€ 1.363 million) and 5.1% in the USA and Japan. Basys cites 7.9% for Europe. According to another study, it is 10% in Germany (Study on the value of medical devices).
In addition to these direct costs, however, there are considerable indirect expenses and follow-up costs to an unknown extent:
- The innovation can be used for more and more indications and applications.
- The innovation treats previously untreatable diseases.
- Better possibilities for diagnosis increase the number of differential diagnoses and thus ensure a more adapted, but also more demanding treatment.
- An earlier diagnosis may increase the duration of treatment.
- Expansion of the concept of illness.
- Medical technology is increasing the number of treatments for various reasons: lower costs of the individual treatment, fewer risks and less pain reduce the inhibition threshold for application. In addition, there are financial interests of the practitioner.
- Medical technology innovations can lead to life extension and thus to additional health expenditure (Pammolli et al.).
Quite apart from the medical benefits for the patient, there are also savings through medical technology devices. Examples: faster and better diagnosis, shorter hospitalization and convalescence, shorter operation times, fewer consultations with doctors (e.g. through telemedicine) and the need for care, lower incapacity for work and retirement. In a series of studies on the savings potential of innovative medical technology, based on around 45 product and process examples, savings potential for the German healthcare system amounting to several billion euros was shown.
See also
- Apparatus medicine
- EN 60601 , series of medical technology standards
literature
- Armin Gärtner: Medical technology and information technology - Vol. 2. - Image management . TÜV-Verlag, Cologne 2005, ISBN 3-8249-0941-3 .
- Trade journal mt - medizintechnik - organ of the VDI medical technology department and organ of the biomedical technology association . TÜV Rheinland, Cologne, six issues a year, ISSN 0344-9416 .
- Joan Costa-Font, Christophe Courbage, Alistair McGuire (eds): The Economics of New Health Technologies. Oxford University Press, Oxford 2009.
- Pammolli F. et al .: Medical devices - Competitiveness and impact on public health expenditure . CERM, Rome.
- Rüdiger Kramme (Hrsg.): Medical technology - procedures - systems - information processing . 3. Edition. Springer Verlag, Heidelberg 2007, ISBN 3-540-34102-1 .
- Armin Gärtner: Medical product safety - Volume 2 - Electrical safety in medical technology, TÜV Media Verlag Cologne 2008, ISBN 978-3-8249-1164-6
- Zauner, Schrempf: Computer Science in Medical Technology - Basics, Software, Computer- Aided Systems , Springer Verlag, Vienna New York, 2009, ISBN 978-3-211-89188-9
- Erich Wintermantel: Medical technology: Life science engineering . 5th edition. Springer Verlag, ISBN 978-3-540-93935-1 .
Individual evidence
- ↑ European Commission: Harmonized Standards: Active implantable medical devices. July 5, 2016, accessed June 16, 2019 .
- ↑ European Commission: Harmonized Standards: In vitro diagnostic medical devices. July 5, 2016, accessed June 16, 2019 .
- ↑ European Commission: Harmonized Standards: Medical devices. July 5, 2016, accessed June 16, 2019 .
- ^ Doctors grow organs from patients' own cells , CNN , April 3, 2006
- ↑ Trial begins for first artificial liver device using human cells , University of Chicago , February 25, 1999
- ^ Mosquera, Mary: Global Medical device market increases just 3 percent in 2012. In: Healthcare Finance News. May 28, 2013, accessed April 25, 2014.
- ^ The European Medical Technology industry in Figures, MedTech Europe, January 2014.
- ↑ SPECTARIS yearbook 2013/2014: The German medical technology industry
- ↑ SPECTARIS - Facts & Figures - German Industry Association for Optical, Medical and Mechatronic Technologies. In: spectaris.de. Retrieved August 28, 2016 .
- ↑ Universities with a degree in "Medical Technology"
- ↑ Olaf Dössel: DGBMT - Innovations in Medical Technology and BioEngineering. In: vde.com. DGBMT, accessed August 28, 2016 .
- ↑ According to the Medical Devices Act (MPG), medical devices are subject to a conformity assessment procedure that is equivalent to official approval.
- ↑ List of previous KOMET participants on the website of the Medical Technology Conference (KOMET)
- ↑ Stella Fuhrer / Peter Frei in: Competence Center Medical Technology
- ^ Gerhard Kocher: Medical technology . In: Gerhard Kocher, Willy Oggier (Ed.): Healthcare Switzerland 2010–2012 - A current overview . 4th edition. Hans Huber, Bern 2010, pp. 221–237
- ^ Department of Medical Technology TU Berlin, Droege & Comp, SPECTARIS, ZVEI: Series of studies on the savings potential of innovative medical technology [1]
Web links
- National information platform for medical technology - BMBF initiative
- BVMed - Federal Association of Medical Technology eV - Medical Technology
- National strategy process "Innovations in medical technology"
- Fachverband Biomedizinische Technik eV
- German Institute for Medical Documentation and Information
- Zentralverband Elektrotechnik- und Elektronikindustrie eV - Association of Electromedical Technology
- SPECTARIS - German Industry Association for Optical, Medical and Mechatronic Technologies eV
- Medical Valley leading edge cluster
- List of all university locations where medical technology can be studied
- Gütegemeinschaft Medizintechnik eV RAL quality mark for medical technology
- Information for developers and designers MED engineering