Sodium-sulfur accumulator
A sodium-sulfur battery , abbreviated NaS battery , NaS battery is a rechargeable battery , a so-called secondary cell . Compared to other types of accumulators, a solid electrolyte and liquid electrodes are used instead of a liquid electrolyte , and high operating temperatures in the range of 270 to 350 ° C are required for operation. The NaS battery belongs to the group of thermal batteries . This type of battery was developed in the late 1970s; By the beginning of 2014, around 180 NaS battery storage power plants with a capacity of 334 MW had been installed worldwide .
General
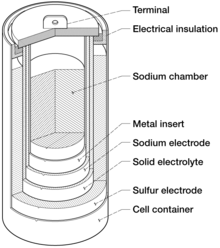
The anode consists of molten sodium , the cathode of a graphite fabric soaked with liquid sulfur . A sodium oxide is used as the electrolyte . Since sodium reacts violently with water, the accumulator must be well protected against environmental influences. Sodium-sulfur accumulators have, in addition to the advantage that the essential basic materials such as sodium, sulfur and aluminum are readily available, a comparatively high storage density in the range of just over 200 Wh / kg.
Experimental applications from the 1980s to the mid-1990s were drive systems for electric cars and energy storage devices in communication satellites . Current applications are small to medium-sized stationary battery storage power plants in Japan, which are used to deliver peak loads and to stabilize the public power grid. In Germany, the Berlin company Younicos has been operating a 1 MW NaS battery together with Vattenfall since 2010 as a pilot project to compensate for volatile renewable energies. In the same year, an even larger NaS battery was installed in Texas to increase the security of supply for an entire small town. In application areas such as electric cars and also as power supply systems in space applications, NaS cells have been replaced by other, more suitable energy storage systems.
As a manufacturer of NaS batteries, only Japanese producers are significant worldwide. As of 2010, the largest and dominant manufacturer in this segment is NGK Insulators , which, together with the energy network operator Tōkyō Denryoku (Tokyo Electric Power, TEPCO ), has been using NaS cells in smaller, stationary battery storage power plants since the early 1990s. Other manufacturers are the Japanese companies Hitachi and GS Yuasa . Former manufacturers of NaS cells for mobile applications include Asea Brown Boveri (electric car), Silent Power Ltd. in England (electric car) and Ford Aerospace in the USA.
functionality
The NaS cell is a high temperature secondary cell. In contrast to many other accumulators , it has a very low electrochemical self-discharge, the efficiency between charge and discharge is in the range of 70 to 85%. The practically non-existent electrochemical self-discharge is put into perspective by the fact that the cell has to be kept in a high temperature range of approx. 300 to 350 ° C in order to maintain its functionality, which requires additional heating systems in addition to appropriate thermal insulation from the cooler environment. If this heating energy required for operation is added to the self-discharge or if the heating power is taken from the battery system, there is a high overall self-discharge in small systems due to the high specific surface area.
As with all systems in which thermal losses are to be reduced, the following applies:
- Small NaS batteries are therefore only suitable for short-term storage of energy.
- However, because of the reciprocal proportionality of the surface to the volume and thus the theoretically possible low thermal losses, super- insulated or very large NaS cells can minimize them to such an extent that the efficiency is greatly improved.
The number of charging and discharging cycles is large compared to other battery types, but, as shown in the figure on the right, it depends heavily on the depth of discharge. If the accumulator is only very slightly discharged in each cycle, which corresponds to a significant reduction in the effective capacity , some 10,000 charging cycles are possible. If, on the other hand, a discharge to 10% is always carried out before recharging, the number is reduced to a few 1000 cycles until failure. The wear and tear of these batteries, which are sensitive to deep discharge, is a consequence of thermal processes in the cell, including thermal runaway, especially in the case of deep discharge .
The high temperature is necessary because sulfur and sodium must be in liquid form. The respective solidification temperatures must be exceeded by far so that a sufficient flow of energy can be established between the electrodes. While the electrodes are in liquid form at high temperatures, the electrolyte in NaS cells is always in solid form. It consists of a ceramic that conducts sodium ions and is also an insulator for electrons . An essential component of the ceramic is sodium β-aluminate (NaAl 11 O 17 ), in which, from a temperature of 270 ° C, the sodium ions become so mobile that there is sufficient conductivity. Further possible materials are, for example, sodium oxide or magnesium oxide .
The US company Ceramatec developed (2009) a version in Utah that also works at lower temperatures. When using a new NaSICON membrane, the accumulator can be operated at 90 ° C. All components remain fixed.
Electrochemistry
During the discharge, sodium oxidizes on the sodium β-aluminate and forms positively charged sodium ions. These ions migrate through the electrolyte and reduce the sulfur to sodium pentasulfide (Na 2 S 5 ) on the positive electrode :
Liquid sodium is oxidized at the negative electrode :
When charging, the processes run in the opposite direction. The overall reaction is then:
The electrochemical reaction depends on factors such as cell design and temperature, the internal resistance is approx. 35 mΩ and is almost independent of the state of charge of the cell. The open circuit voltage of a charged NaS cell is 2.076 V, with this voltage remaining almost constant up to a 65% discharge if sodium pentasulphide (Na 2 S 5 ) is predominantly present .
After the sulfur has been consumed, part of the sodium pentasulphide is reduced to various forms of sodium polysulphide (Na 2 S 5-x} ) in the area of deep discharge .
Thereafter, with increasing formation of the various sodium polysulphides, the cell voltage drops almost linearly up to the final discharge voltage of 1.78 or 1.9 V. At a final discharge voltage of 1.9 V, Na 2 S 4 is primarily present; at 1.78 V, Na 2 S 3 is present. In the event of further deep discharge, which is harmful to the accumulator, Na 2 S 2 forms in the cell , which is undesirable because it leads to a high internal resistance and thus large thermal losses in the cell. The thermal load can damage the cell.
Technical specifications
The following table summarizes the technical data of some of the NaS cells currently available on the market from Japanese production. The design is exclusively an elongated, cylindrical shape.
| Manufacturer | Type | Capacity [Ah] | Diameter [mm] | Length [mm] | Weight [kg] | Specific energy [Wh / kg] |
|---|---|---|---|---|---|---|
| NGK insulators | T4.1 | 160 | 62 | 375 | 2 | 160 |
| NGK insulators | T4.2 | 248 | 68 | 390 | 2.4 | 202 |
| NGK insulators | T5 | 632 | 91 | 515 | 5.4 | 226 |
| GS Yuasa | 176 | 64 | 430 | 2.7 | 120 | |
| Hitachi | 280 | 75 | 400 | 4th | 133 |
literature
- Michael Sterner , Ingo Stadler (ed.): Energy storage. Need, technologies, integration. 2nd edition, Berlin Heidelberg 2017, ISBN 978-3-662-48893-5 .
- Bernhard Hauck: Electronic monitoring and control devices to maintain the current quality of multi-cell electrochemical storage systems . Kaiserslautern 2003, DNB 969874197 , urn : nbn: de: bsz: 386-kluedo-16560 (habilitation thesis, TU Kaiserslautern).
- Jeffrey W. Braithwaite, William L. Auxer: Handbook of Batteries . Ed .: David Linden. 3. Edition. McGraw-Hill, 2002, ISBN 0-07-135978-8 , Chapter 40: Sodium-Beta Batteries .
proof
- ↑ a b Patent US3982959 : Sodium-sulfur battery cells. Published September 28, 1976 , Inventors: Bernard Austin Partridge, Thomas Rhys Jenkins, Michael McGuire.
- ↑ Katja Buß et al .: Global Distribution of Grid-connected Electrical Energy Storage Systems . In: International Journal of Sustainable Energy Planning and Management . tape 9 , 2016, p. 31–56 , doi : 10.5278 / ijsepm.2016.9.4 .
- ↑ Sodium-sulfur battery. Heidjann GmbH, accessed on April 15, 2018 .
- ↑ Sodium-sulfur accumulator. Spektrum der Wissenschaft Verlagsgesellschaft mbH, accessed on April 15, 2018 (Lexicon of Chemistry).
- ↑ K. Takashima et al: The Sodium Sulfur Battery for a 1 MW, 8 MWh Load Leveling System. Proceedings if the International Conference on Batteries for Utility Energy Storage, March 1991, pp. 333-349.
- ^ AA Koenig, JR Rasmussen: Development of a high specific power sodium sulfur cell . In: IEEE (Ed.): Proceedings of the 34th International Power Sources Symposium . 1990, p. 30-33 , doi : 10.1109 / IPSS.1990.145783 .
- ↑ William Auxer: The PB sodium sulfur cell for satellite battery applications . In: Proceedings of the 32nd International Power Sources Symposium . 1986, p. 49-54 , bibcode : 1986poso.symp ... 49A .
- ↑ Younicos - short-term storage for system stability
- ↑ Texas Town Installs a Monster Battery For Backup Power. on: popsci.com , May 4, 2010.
- ↑ a b c Jeffrey W. Braithwaite, William L. Auxer: Handbook of Batteries . 2nd Edition. McGraw-Hill, 2002, ISBN 0-07-135978-8 , Chapter 40: Sodium-Beta Batteries , pp. 40.1-40.31 .
- ^ Sodium Sulfur Battery Energy Storage ( Memento from April 18, 2011 in the Internet Archive ). Xcel Energy Fund, 2010.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 .
- ↑ American Ceramic Society: Ceramatec