Nucleoside-modified mRNA
Nucleoside-modified messenger RNA (modRNA) is a synthetic, chemically modified messenger ribonucleic acid (mRNA) in which individual nucleosides are replaced by other naturally modified nucleosides or by synthetic nucleoside analogs . modRNA is used experimentally or therapeutically to induce the production of a desired protein in certain cells . An important area of application is the manufacture of vaccines against SARS-CoV-2 .
requirements
In a cell , mRNA is produced by synthesizing a ribonucleic acid (RNA) strand from a deoxyribonucleic acid (DNA) template, with the codogenic strand section serving as a template . This process is known as transcription . The mRNA is then read on ribosomes and in turn serves as a blueprint for the synthesis of proteins by specifying their amino acid sequence . The process of protein synthesis described last is called translation .
Principle of nucleoside modification
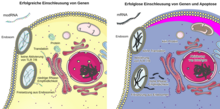
If you want to induce cells to synthesize proteins that they normally do not make, you can use modRNA whose nucleotide sequence encodes the amino acid sequence of these proteins . The mRNA synthesized in vitro must then be introduced into the organism, for example injected, absorbed into the target cells and read there. In this way, translation takes place without prior transcription . A blueprint for foreign proteins is smuggled into the cells, so to speak. In order to achieve this goal, one has to bypass systems that exist in the human organism to prevent the penetration and translation of foreign mRNA. On the one hand, there are enzymes ( ribonucleases ) that break down “normal”, i.e. unmodified, mRNA. On the other hand, there are also intracellular barriers against foreign mRNA. When single-stranded RNA ( ssRNA ) is absorbed through the cell membrane in endosomes , it is recognized by toll-like receptors 7 and 8, which are part of the innate immune system . Ultimately, this leads to the protein synthesis in the cell being switched off, interferons and cytokines being released and programmed cell death ( apoptosis ) to occur via the activation of the transcription factors TNF-alpha and AP-1 . This can be avoided by modifying the system for in-vitro production of the mRNA so that instead of the physiological nucleoside uridine, the similar (also naturally occurring) pseudouridine (Ψ) or N 1 -methyl pseudouridine (m1Ψ) or instead of cytosine the 5-methyl-cytosine are incorporated. N 1 -methyl-pseudouridine and 5-methyl-cytosine do not occur naturally. If an mRNA contains one or two of these modified nucleosides, this leads to a change in the secondary structure , which on the one hand prevents it from being recognized by the innate immune system, but on the other still allows an effective translation to a protein.
Importance of untranslated regions
A normal mRNA begins and ends with sections that do not code for amino acids of the actual protein. These sequences at the 3 ' and 5' ends of an mRNA strand are referred to as untranslated regions (UTRs). The two UTRs at their strand ends are essential for the stability of an mRNA and a modRNA as well as for the efficiency of translation, i.e. for the amount of protein produced. By selecting suitable UTRs for the synthesis of a modRNA, one can optimize the production of the target protein in the target cells.
Obstacles, use of nanoparticles
If you want to smuggle modRNA into certain target cells, you face various difficulties. On the one hand, you have to protect the modRNA from ribonucleases . This can be done, for example, by packing them in lipid nanoparticles (solid lipid nanoparticles). Such “packaging” can also help ensure that the modRNA is absorbed into the target cells. This is useful when used in vaccines , for example , as nanoparticles are taken up by dendritic cells and macrophages , both of which play an important role in activating the immune system.
Furthermore, it can be desirable that the applied modRNA is specifically introduced into certain body cells. This is the case, for example, when you want to stimulate heart muscle cells to multiply. The packaged modRNA can then be injected directly intra-arterially into the coronary arteries , for example .
Risks
If the modRNA does not get into the target cells but into other cells, undesirable effects can occur. For example, if the encoded protein is actually supposed to stimulate myocardial cells to multiply, but is incorrectly produced in other cells, this could lead to growths. However, such a negative effect is limited in time by the fact that the modRNA, despite its increased stability compared to normal mRNA, is ultimately broken down, as are the proteins it encodes.
It could be objected that changes in the genome of the cells, i.e. mutations , could be triggered with consequences up to the development of cancer. First of all, it should be considered that the genetic information is present as DNA (not as RNA) in the cell nucleus , and modRNA does not get into the cell nucleus. In addition, there is physiologically no reverse transcriptase in the human body , i.e. no enzyme that can transcribe (transcribe) mRNA into DNA. Here again the objection is made that there are infections in humans with viruses that form reverse transcriptases (for example HIV ) and that it is precisely these reverse transcriptases that could lead to reverse transcription of the modRNA. However, these reverse transcriptases of viruses are highly specific and only transcribe the virus' own RNA, so this problem can probably be neglected.
Areas of application
The currently most important application of modRNA is the production of vaccines against SARS-CoV-2 . The vaccines, which are developed by the cooperation of the companies Biontech / Pfizer / Fosun International ( Tozinameran ) as well as by the companies Curevac ( CVnCoV ) and Moderna ( mRNA-1273 ) as well as by other companies as protection against COVID-19 disease, work with modRNA technologies. The vaccines from the companies Biontech / Pfizer / Fosun International and Moderna are approved for use in some countries such as the USA or the member states of the European Union and are used or should be used promptly.
Further possibilities of using modRNA are the regeneration of damaged heart muscle tissue and cancer therapy.
literature
- Kenneth R. Chien, Lior Zangi, Kathy O. Lui: Synthetic Chemically Modified mRNA (modRNA): Toward a New Technology Platform for Cardiovascular Biology and Medicine . In: Cold Spring Harbor Perspectives in Medicine . tape 5 , no. 1 , January 1, 2015, ISSN 2157-1422 , p. a014035 , doi : 10.1101 / cshperspect.a014035 , PMID 25301935 , PMC 4292072 (free full text) - ( Online [accessed November 28, 2020]).
Individual evidence
- ↑ Ajit Magadum, Keerat Kaur, Lior Zangi: mRNA-Based Protein Replacement Therapy for the Heart . In: Molecular Therapy . tape 27 , no. 4 , April 10, 2019, ISSN 1525-0016 , p. 785–793 , doi : 10.1016 / j.ymthe.2018.11.018 , PMID 30611663 , PMC 6453506 (free full text) - ( cell.com [accessed December 13, 2020]).
- ↑ Kenneth R. Chien, Lior Zangi, Kathy O. Lui: Synthetic Chemically Modified mRNA (modRNA): Toward a New Technology Platform for Cardiovascular Biology and Medicine . In: Cold Spring Harbor Perspectives in Medicine . tape 5 , no. 1 , January 1, 2015, ISSN 2157-1422 , p. a014035 , doi : 10.1101 / cshperspect.a014035 , PMID 25301935 , PMC 4292072 (free full text) - ( Online [accessed November 28, 2020]).
- ↑ Nishat Sultana, Ajit Magadum, Yoav Hadas, Jason Kondrat, Neha Singh: Optimizing Cardiac Delivery of Modified mRNA . In: Molecular Therapy . tape 25 , no. 6 , June 7, 2017, ISSN 1525-0016 , p. 1306-1315 , doi : 10.1016 / j.ymthe.2017.03.016 , PMID 28389322 , PMC 5474881 (free full text) - ( cell.com [accessed December 24, 2020]).
- ↑ Katalin Karikó, Michael Buckstein, Houping Ni, Drew Weissman: Suppression of RNA Recognition by Toll-like Receptors: The Impact of Nucleoside Modification and the Evolutionary Origin of RNA . In: Immunity . tape 23 , no. 2 , August 2005, ISSN 1074-7613 , p. 165-175 , doi : 10.1016 / j.immuni.2005.06.008 .
- ↑ Alexandra G. Orlandini von Niessen, Marco A. Poleganov, Corinarechner, Arianne Plaschke, Lena M. Kranz: Improving mRNA-Based Therapeutic Gene Delivery by Expression-Augmenting 3 ′ UTRs Identified by Cellular Library Screening . In: Molecular Therapy . tape 27 , no. 4 , April 10, 2019, ISSN 1525-0016 , p. 824–836 , doi : 10.1016 / j.ymthe.2018.12.011 , PMID 30638957 , PMC 6453560 (free full text) - ( cell.com [accessed November 28, 2020]).
- ^ Nanoparticle vaccines . In: Vaccine . tape 32 , no. 3 , January 9, 2014, ISSN 0264-410X , p. 327–337 , doi : 10.1016 / j.vaccine.2013.11.069 ( online [accessed November 28, 2020]).
- ↑ Keerat Kaur, Lior Zangi: Modified mRNA as a Therapeutic Tool for the Heart . In: Cardiovascular Drugs and Therapy . tape 34 , no. 6 , December 1, 2020, ISSN 1573-7241 , p. 871-880 , doi : 10.1007 / s10557-020-07051-4 , PMID 32822006 , PMC 7441140 (free full text).
- ↑ Uli Blumenthal, Michael Lange: Corona vaccines from Biontech / Pfizer and Moderna: How mRNA vaccines work (interview with Leif Erik Sander). In: Deutschlandfunk. November 22, 2020, accessed December 2, 2020 .
- ↑ Fee Anabelle Riebeling: Corona fact check: 7 things that make vaccine skeptics stomach ache. In: 20 minutes. November 18, 2020, accessed November 28, 2020 .
- ↑ Kristina Kreisel: "The Corona Explainers": Corona vaccines change DNA? This is what the experts say about the excitement theory. In: Focus Online. November 19, 2020, accessed November 28, 2020 .
- ↑ Christina Hohmann-Jeddi: Hope BNT162b2: How do mRNA vaccines work? In: Pharmaceutical newspaper. November 10, 2020, accessed November 28, 2020 .
- ↑ n-tv NEWS: Paul Ehrlich Institute approves corona vaccine. Retrieved December 25, 2020 .
- ↑ Annette B. Vogel, Isis Kanevsky, Ye Che, Kena A. Swanson, Alexander Muik: A prefusion SARS-CoV-2 spike RNA vaccine is highly immunogenic and prevents lung infection in non-human primates . In: bioRxiv . September 8, 2020, p. 2020.09.08.280818 , doi : 10.1101 / 2020.09.08.280818 ( online [accessed November 28, 2020]).
- ↑ COVID-19: About CureVac's Development of an mRNA-Based Vaccine. In: curevac.com. CureVac, November 28, 2020, accessed November 28, 2020 .
- ↑ Moderna's Pipeline. In: modernatx.com. Moderna, accessed on November 28, 2020 .
- ↑ Florian Krammer: SARS-CoV-2 vaccines in development . In: Nature . tape 586 , no. 7830 , October 2020, ISSN 1476-4687 , p. 516-527 , doi : 10.1038 / s41586-020-2798-3 ( online [accessed November 28, 2020]).
- ↑ Keerat Kaur, Lior Zangi: Modified mRNA as a Therapeutic Tool for the Heart . In: Cardiovascular Drugs and Therapy . tape 34 , no. 6 , December 1, 2020, ISSN 1573-7241 , p. 871-880 , doi : 10.1007 / s10557-020-07051-4 , PMID 32822006 , PMC 7441140 (free full text).
- ^ Lior Zangi, Kathy O. Lui, Alexander von Gise, Qing Ma, Wataru Ebina: Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction . In: Nature Biotechnology . tape 31 , no. October 10 , 2013, ISSN 1546-1696 , p. 898–907 , doi : 10.1038 / nbt.2682 , PMID 24013197 , PMC 4058317 (free full text) - ( online [accessed November 28, 2020]).
- ↑ Ugur Sahin, Özlem Türeci: Personalized vaccines for cancer immunotherapy . In: Science . tape 359 , no. 6382 , March 23, 2018, ISSN 0036-8075 , p. 1355–1360 , doi : 10.1126 / science.aar7112 ( online [accessed November 28, 2020]).