Great-like receptors
Toll-like receptors (TLR briefly, English toll-like receptor ) are structures of the innate immune system (innate immunity) and belong to a group of receptors , the PRRs (Pattern-Recognition Receptors) . They are used to recognize PAMPs (Pathogen-Associated Molecular Patterns) , which are structures that occur exclusively on or in pathogens , and control the corresponding activation of genes . This initiates and modulates the activation of the “antigen-specific acquired immune system” (antigen-specific acquired immunity) . The innate defense system is able to distinguish between “self” and “not self” through the “toll-like receptors”. TLRs are generally expressed in dendritic cells and macrophages, with little to no expression in epithelial cells.
The name toll-like receptor (referred to in German-language literature as signal transduction- mediating PRRs or rarely also as toll-like receptor ) is derived from a protein in Drosophila melanogaster , which the research group around Nobel Prize winner Christiane Nüsslein-Volhard was so enthusiastic about was that she humorously called it great after the German expression . The moment that gave the great gene of the fruit fly its name was when she was sitting across from her colleague Eric Wieschaus at a double microscope that allows two people to examine the same object at the same time. “One day when we saw a mutant embryo whose development was ventralized, we were both completely surprised and spontaneously shouted 'great'. Until then we only knew dorsalized embryos ”. TLRs consist of proteins that resemble Toll, i.e. are toll-like .
Since the discovery of the first toll-like receptor in the mid-1990s, new variants have been discovered again and again in humans and animals . TLRs are found in all vertebrates but also in simpler organisms , such as Drosophila melanogaster , which suggests that this is an evolutionarily very old system. Most species have more than ten different (known) TLRs, some types e.g. B. occur in the mouse , but not in humans.
TLRs recognize various functional components of viruses , bacteria and fungi and can thus trigger biochemical reaction chains in the cells that serve to defend themselves against these pathogens.
Discovery of TLRs
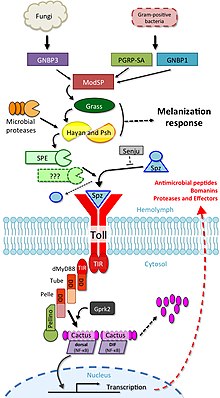
When microorganisms were first identified as the cause of infectious diseases , it was clear that multicellular organisms must be able to recognize them, and that in order to do this, it is necessary to recognize molecular structures typical of microorganisms. A large body of literature, spanning much of the 20th century, is devoted to key molecules and their receptors. More than 100 years ago, Richard Pfeiffer , a student of Robert Koch , coined the term ' endotoxin ' to name a substance that was produced by gram-negative bacteria and that led to fever and shock in animal experiments. In the decades that followed, the endotoxins were chemically characterized and identified as lipopolysaccharides (LPS), which are produced by most gram-negative bacteria. It has been shown that other molecules (bacterial lipopeptides, flagellins and unmethylated DNA ) also lead to an immune response . Logically it was concluded from this that there must be receptors that are able to induce an immune response for such molecular structures. However, these were not found for many years.
In the mid-1990s, research in the developmental biology of Drosophila melanogaster discovered by chance that toll-negative mutants are very susceptible to fungal attack. This observation initiated a targeted search for similar proteins in mammalian cells. In 1994 Nomura and colleagues were able to find the first human TLR, which Taguchi and colleagues were able to assign to a chromosome in 1996 . This shows that the immune response mediated via toll-like receptors is an evolutionarily very old form that is genetically highly conserved. Since the role of TLRs in immune defense was not yet known at the time, it was assumed that TLR1 would play a role in mammalian developmental biology. In 1997, Charles Janeway and Ruslan Medzhitov showed that a toll-like receptor, when artificially bound to appropriate antibodies, can activate certain genes that are necessary for an adaptive immune response. The role of TLR4 as an LPS receptor was discovered by Bruce A. Beutler and colleagues. Over time, the ligands of the other TLRs were also determined. Shizuo Akira played a central role in this.
Structure and ligands
The common structural features of all toll-like receptors are the N-terminal leucine-rich LRR sequences ( leucine-rich repeats ) and the TIR domain (Toll / IL-1R homology domain) . The different TLRs can each identify different PAMPs (Pathogen Associated Molecular Patterns) through direct interaction with the respective membrane surface of the pathogen. TLR3, TLR7, TLR8 and TLR9 are located on the membrane of endosomes or endolysosomes, while the remaining toll-like receptors are located on the cell membrane.
TLR2 recognizes many components of bacteria, mycoplasma, fungi and viruses. This also includes the lipoproteins of bacteria and mycoplasmas. The detection takes place in that TLR2 forms a heterodimer with either TLR1 or TLR6. The resulting TLR1 / TLR2 and TLR6 / TLR2 complexes recognize triacyl and diacyllipoproteins. The activation of TLR2 leads to the release of a number of cytokines . Alpha interferon (IFN-α) is only partially released. It is generally assumed that the release of IFN-α depends on the cell type. TLR10 is similar in its primary structure (sequence match) to TLR1 and TLR6, but the ligand is not yet known. TLR4 can recognize lipopolysaccharide (LPS) on the cell surface along with MD2 (myeloid differentiation factor 2) . LPS is a substance from the outer membrane of gram-negative bacteria and is used in animal models for the limited simulation of acute infections. In the detection of LPS, two TLR-4-MD2-LPS complexes work together to form a TLR-4 homodimer.
TLR5 is mainly expressed in the lamina propria , where it recognizes bacterial flagellin. As an immune response, TLR5 induces the differentiation of B cells into IgA-producing blood cells and of T cells into antigen-specific Th17 and Th1 cells. TLR11, which only occurs in mice but not in humans, shows great similarities to TLR5. It recognizes a profilin-like molecule derived from the intracellular protozoan Toxoplasma gondii . A number of TLRs, including TLR3, TLR7, TLR8, and TLR9, recognize nucleic acids derived from viruses or bacteria. Activation of these TLRs leads to the formation of alpha interferon and other inflammatory cytokines . TLR3 detects viral double-stranded RNA in the endolysosome. The dsRNA binds to the N-terminal and the C-terminal end of the LRR sequence.
| receptor | Ligands | Origin of the ligands | Adapter protein of the TLR | Cellular localization | Cell types |
|---|---|---|---|---|---|
| TLR 1 | Various triacyl lipopeptides | Bacterial lipoprotein | MyD88 / MAL | Cell surface |
|
| TLR 2 | Various glycolipids | Bacterial peptidoglycans | MyD88 / MAL | Cell surface |
|
| Various lipopeptides and proteolipids | Bacterial peptidoglycans | ||||
| Lipoteichoic acid | Gram positive bacteria | ||||
| HSP70 | Host cells | ||||
| Zymosan ( beta-glucan ) | Mushrooms | ||||
| Numerous others | |||||
| TLR 3 | Double stranded RNA , poly I: C | Viruses | TRIF | Cell compartment |
|
| TLR 4 | Lipopolysaccharide , Eritoran | Gram negative bacteria | MyD88 / MAL / TRIF / TRAM | Cell surface |
|
| Multiple heat shock proteins | Bacteria and host cells | ||||
| Fibrinogen | Host cells | ||||
| Heparan sulfate fragments | Host cells | ||||
| Hyaluronic acid fragments | Host cells | ||||
| nickel | |||||
| Various opioids | |||||
| TLR 5 | Bacterial flagellin | bacteria | MyD88 | Cell surface |
|
| Profilin | Toxoplasma gondii | ||||
| TLR 6 | Various diacyl lipopeptides | Mycoplasma | MyD88 / MAL | Cell surface |
|
| TLR 7 | Imidazoquinoline | Low molecular weight synthetic substances | MyD88 | Cell compartment |
|
| Loxoribine (a guanosine analog ) | |||||
| Bropirimine | |||||
| Imiquimod , resiquimod | |||||
| Single stranded RNA | RNA viruses | ||||
| TLR 8 | Low molecular weight synthetics, single-stranded viral RNA, resiquimod, phagocytosed bacterial RNA | MyD88 | Cell compartment |
|
|
| TLR 9 | Unmethylated CpG oligonucleotides (DNA) | Bacteria, dna viruses | MyD88 | Cell compartment |
|
| TLR 10 | Triacylated lipopeptides | unknown | Cell surface |
|
|
| TLR 11 | Profilin | Toxoplasma gondii | MyD88 | Cell compartment |
|
| TLR 12 | Profilin | Toxoplasma gondii | MyD88 | Cell compartment |
|
| TLR 13 | Bacterial ribosomal RNA sequence CGGAAAGACC (unmethylated) | Viruses, bacteria | MyD88, TAK-1 | Cell compartment |
|
The intracellular signal cascade
The chemoattractor proteins "C3a" and "C5a" of the complement system arise through proteolytic cleavage from the inactive precursors C3 and C5, respectively. When activated, they attract macrophages and neutrophil granulocytes . These phagocytes have TLR-type receptors on their surface. The TLRs react to bacterial proteoglycans or lipopolysaccharides (LPS), DNA and RNA. They trigger a signal cascade in their carrier cells that ultimately stimulates the defense against infection. After recognition of these bacterial surface structures on the extracellular side, intracellular signal cascades are triggered by TLR.
The recognition of PAMPs (Pathogen Associated Molecular Patterns) by TLRs leads to an increase in the transcription rate of certain genes, depending on which TLRs and which cell types are involved. The difference between the signal cascades activated by the individual TLRs can be explained at least in part by the TIR domain-containing adapter molecules (TIR domain-containing adapter molecules). There are five TIR domain-containing adapter molecules, including MyD88, TRIF / TICAM-1 (TIR-domain-containing adapter inducing IFN-β), TIRAP / Mal, TRAM (TRIF-related adapter molecule), and SARM (sterile alpha and Armadillo motif-containing protein). TLR signal chains are roughly divided into two different reaction chains depending on the involvement of the adapter molecules MyD88 and TRIF.
This leads to phosphorylation and thus activation of intracellular kinases , the task of which is the phosphorylation of intracellular inhibitors of transcription factors . First, the adapter protein MyD88 binds to the cytoplasmic section of the TLR. As a result, the IL-1 receptor-associated kinase (IRAK) now binds to MyD88 and activates itself through autophosphorylation. After further individual steps, the transcription factor NF-κB is activated , which then translocates into the cell nucleus and expresses it there Regulates genes for TNFα, IL-1, IL-12 and E-selectin.
literature
- K. Takeda, S. Akira: Toll-like receptors in innate immunity. In: International immunology , Volume 17, Number 1, January 2005, ISSN 0953-8178 , pp. 1-14, doi: 10.1093 / intimm / dxh186 , PMID 15585605 (review).
- Luke AJ O'Neill: The Immunological Early Warning System. Spectrum of Science , August 2005, pp. 68–75
- SE Turvey, TR Hawn: Towards subtlety: understanding the role of Toll-like receptor signaling in susceptibility to human infections. In: Clinical immunology (Orlando FL), Volume 120, No. 1, July 2006, ISSN 1521-6616 , pp. 1-9, doi: 10.1016 / j.clim.2006.02.003 , PMID 16563867 (review).
- Nicola Siegmund-Schultze: Deutsches Ärzteblatt: Toll-like receptors: New target structure for immune-stimulating drugs . In: Deutsches Ärzteblatt . tape 104 , no. 16 . Deutscher Ärzte-Verlag, April 20, 2007, p. A-1072 / B-954 / C-908 .
Individual evidence
- ↑ a b c d Peter Fritsch: Dermatology and Venereology . 2nd Edition. Springer Verlag, 2004, ISBN 3-540-00332-0 .
- ^ Toll-like Receptor , German. Doctor Bl 2007; 104 (16): A-1072 / B-954 / C-908. Retrieved June 8, 2020.
- ↑ B. Lemaitre, J. Hoffmann: The host defense of Drosophila melanogaster . In: Annual Review of Immunology . 25, 2007, pp. 697-743. doi : 10.1146 / annurev.immunol.25.022106.141615 . PMID 17201680 .
- ↑ S. Valanne, JH Wang, M. Rämet: The Drosophila Toll signaling pathway . In: Journal of Immunology . 186, No. 2, January 2011, pp. 649-656. doi : 10.4049 / jimmunol.1002302 . PMID 21209287 .
- ↑ JP Dudzic, MA Hanson, I. Iatsenko, S. Kondo, B. Lemaitre: More Than Black or White: Melanization and Toll Share Regulatory Serine Proteases in Drosophila . In: Cell Reports . 27, No. 4, April 2019, pp. 1050-1061.e3. doi : 10.1016 / j.celrep.2019.03.101 . PMID 31018123 .
- ^ MA Hanson, PT Hamilton, SJ Perlman: Immune genes and divergent antimicrobial peptides in flies of the subgenus Drosophila . In: BMC Evolutionary Biology . 16, No. 1, October 2016, p. 228. doi : 10.1186 / s12862-016-0805-y . PMID 27776480 .
- ↑ B. Lemaitre, E. Nicolas, L. Michaut, JM Reichhart, JA Hoffmann : The dorsoventral regulatory gene cassette spätzle / Toll / cactus controls the potent antifungal response in Drosophila adults. In: Cell . Volume 86, Number 6, September 1996, ISSN 0092-8674 , pp. 973-983, PMID 8808632 .
- ↑ T. Kawai, S. Akira: The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. In: Nature Immunology . Volume 11, Number 5, May 2010, pp. 373-384, doi: 10.1038 / ni.1863 , PMID 20404851 (review).
- ↑ a b c The source is, unless stated otherwise in the table entries : C. Waltenbaugh, T. Doan, R. Melvold, S. Viselli: Immunology (= Lippincott's Illustrated reviews). Wolters Kluwer Health / Lippincott Williams & Wilkins, Philadelphia 2008, ISBN 978-0-7817-9543-2 , p. 17.
- ↑ a b I. Sabroe, SK Dower, MK Whyte: The role of Toll-like receptors in the regulation of neutrophil migration, activation, and apoptosis . In: Clinical Infectious Diseases . 41 Suppl 7, November 2005, pp. S421-426. doi : 10.1086 / 431992 . PMID 16237641 .
- ↑ a b c d F. Sallusto, A. Lanzavecchia: The instructive role of dendritic cells on T-cell responses . In: Arthritis Research . 4 Suppl 3, 2002, pp. S127-132. doi : 10.1186 / ar567 . PMID 12110131 . PMC 3240143 (free full text).
- ↑ CF Nicodemus, JS Berek: TLR3 agonists as immunotherapeutic agents. In: Immunotherapy. Volume 2, number 2, March 2010, pp. 137-140, doi: 10.2217 / imt.10.8 , PMID 20635920 .
- ↑ KA Shirey, W. Lai et al. a .: The TLR4 antagonist Eritoran protects mice from lethal influenza infection. In: Nature. Volume 497, number 7450, May 2013, ISSN 1476-4687 , pp. 498-502, doi: 10.1038 / nature12118 , PMID 23636320 , PMC 3725830 (free full text).
- ^ EJ Hennessy, AE Parker, LA O'Neill: Targeting Toll-like receptors: emerging therapeutics? In: Nature reviews. Drug discovery. Volume 9, Number 4, April 2010, ISSN 1474-1784 , pp. 293-307, doi: 10.1038 / nrd3203 , PMID 20380038 (review).
- ↑ S. Gerondakis, RJ Grumont, A. Banerjee: Regulating B-cell activation and survival in response to TLR signal . In: Immunology and Cell Biology . 85, No. 6, 2007, pp. 471-475. doi : 10.1038 / sj.icb.7100097 . PMID 17637697 .
- ^ E. Cario, IM Rosenberg, SL Brandwein, PL Beck, HC Reinecker, DK Podolsky: Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors . In: Journal of Immunology . 164, No. 2, January 2000, pp. 966-972. doi : 10.4049 / jimmunol.164.2.966 . PMID 10623846 .
- ↑ M. Peana, K. Zdyb, S. Medici, A. Pelucelli, G. Simula, E. Gumienna-Kontecka, MA Zoroddu: Ni (II) interaction with a peptide model of the human TLR4 ectodomain . In: Journal of Trace Elements in Medicine and Biology . 44, December 2017, pp. 151-160. doi : 10.1016 / j.jtemb.2017.07.006 . PMID 28965571 .
- ↑ RM Salazar Gonzalez, H. Shehata, MJ O'Connell, Y. Yang, ME Moreno-Fernandez, CA Chougnet, J. Aliberti: Toxoplasma gondii- derived profilin triggers human toll-like receptor 5-dependent cytokine production . In: Journal of Innate Immunity . 6, No. 5, 2014, pp. 685-694. doi : 10.1159 / 000362367 . PMID 24861338 . PMC 4141014 (free full text).
- ↑ C. Maisonneuve, S. Bertholet u. a .: Unleashing the potential of NOD and Toll-like agonists as vaccine adjuvants. In: Proceedings of the National Academy of Sciences . Volume 111, number 34, August 2014, ISSN 1091-6490 , pp. 12294-12299, doi: 10.1073 / pnas.1400478111 , PMID 25136133 , PMC 4151741 (free full text) (review).
- ↑ Y. Guan, DR Ranoa, S. Jiang, SK Mutha, X. Li, J. Baudry, RI Tapping: Human TLRs 10 and 1 share common mechanisms of innate immune sensing but not signaling . In: Journal of Immunology (Baltimore, Md.: 1950) . 184, No. 9, May 2010, pp. 5094-5103. doi : 10.4049 / jimmunol.0901888 . PMID 20348427 .
- ↑ T. Chuang, RJ Ulevitch: Identification of hTLR10: a novel human Toll-like receptor preferentially expressed in immune cells . In: Biochimica Et Biophysica Acta . 1518, No. 1-2, March 2001, pp. 157-161. doi : 10.1016 / s0167-4781 (00) 00289-x . PMID 11267672 .
- ^ V. Hornung, S. Rothenfusser, S. Britsch, A. Krug, B. Jahrsdörfer, T. Giese, S. Endres, G. Hartmann: Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides . In: Journal of Immunology . 168, No. 9, Baltimore, Md.: 1950, May 2002, pp. 4531-4537. doi : 10.4049 / jimmunol.168.9.4531 . PMID 11970999 .
- ^ A b T. Regan, K. Nally, R. Carmody, A. Houston, F. Shanahan, J. Macsharry, E. Brint: Identification of TLR10 as a key mediator of the inflammatory response to Listeria monocytogenes in intestinal epithelial cells and macrophages . In: Journal of Immunology . 191, No. 12, December 2013, pp. 6084-6092. doi : 10.4049 / jimmunol.1203245 . PMID 24198280 .
- ↑ F. Yarovinsky, D. Zhang, JF Andersen, GL Bannenberg, CN Serhan, MS Hayden, S. Hieny, FS Sutterwala, RA Flavell, S. Ghosh, A. Sher: TLR11 activation of dendritic cells by a protozoan profilin-like protein . In: Science . 308, No. 5728, June 2005, pp. 1626-1629. bibcode : 2005Sci ... 308.1626Y . doi : 10.1126 / science.1109893 . PMID 15860593 .
- ↑ R. Pifer, A. Benson, CR Sturge, F. Yarovinsky: UNC93B1 is essential for TLR11 activation and IL-12-dependent host resistance to Toxoplasma gondii . In: The Journal of Biological Chemistry . 286, No. 5, February 2011, pp. 3307-3314. doi : 10.1074 / jbc.M110.171025 . PMID 21097503 . PMC 3030336 (free full text).
- ↑ AA Koblansky, D. Jankovic, H. Oh, S. Hieny, W. Sungnak, R. Mathur, MS Hayden, S. Akira, A. Sher, S. Ghosh: Recognition of profilin by Toll-like receptor 12 is critical for host resistance to Toxoplasma gondii . In: Immunity . 38, No. 1, January 2013, pp. 119-130. doi : 10.1016 / j.immuni.2012.09.016 . PMID 23246311 . PMC 3601573 (free full text).
- ↑ BB Mishra, UM Gundra, JM Teale: Expression and distribution of Toll-like receptors 11-13 in the brain during murine neurocysticercosis . In: Journal of Neuroinflammation . 5, December 2008, p. 53. doi : 10.1186 / 1742-2094-5-53 . PMID 19077284 . PMC 2631477 (free full text).
- ↑ Z. Shi, Z. Cai, A. Sanchez, T. Zhang, S. Wen, J. Wang, J. Yang, S. Fu, D. Zhang: A novel Toll-like receptor that recognizes vesicular stomatitis virus . In: The Journal of Biological Chemistry . 286, No. 6, February 2011, pp. 4517-4524. doi : 10.1074 / jbc.M110.159590 . PMID 21131352 . PMC 3039399 (free full text).
- ↑ M. Oldenburg, A. Krüger, R. Ferstl, A. Kaufmann, G. Nees, A. Sigmund, B. Bathke, H. Lauterbach, M. Suter, S. Dreher, U. Koedel, S. Akira, T Kawai, J. Buer, H. Wagner, S. Bauer, H. Hochrein, CJ Kirschning: TLR13 recognizes bacterial 23S rRNA devoid of erythromycin resistance-forming modification . In: Science . 337, No. 6098, August 2012, pp. 1111-1115. bibcode : 2012Sci ... 337.1111O . doi : 10.1126 / science.1220363 . PMID 22821982 .
- ↑ H. Hochrein, CJ Kirschning: Bacteria evade immune recognition via TLR13 and binding of their 23S rRNA by MLS antibiotics by the same mechanisms . In: Oncoimmunology . 2, No. 3, March 2013, p. E23141. doi : 10.4161 / onci.23141 . PMID 23802068 . PMC 3661153 (free full text).

