Opipramol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
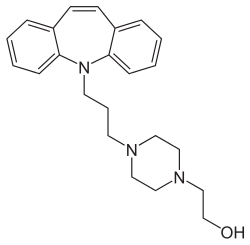
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Opipramol | |||||||||||||||||||||
| other names |
4- [3- (5 H -dibenz [ b , f ] -azepin-5-yl) -propyl] -1-piperazineethanol ( IUPAC ) |
|||||||||||||||||||||
| Molecular formula | C 23 H 29 N 3 O | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 363.50 g mol −1 | |||||||||||||||||||||
| Melting point | ||||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data |
|
|||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Opipramol is a calming , mood-lifting , anxiety and tension - relieving drug .
It belongs to the group of tricyclic antidepressants , but it differs significantly from their usual mode of action ( see section pharmacology ).
Opipramol is a frequently prescribed psychotropic drug in Germany (as of 2016). The calming effect occurs before the mood-lifting effect sets in.
Chemically, Opipramol belongs to the class of dibenzazepines and is used medicinally in the form of Opipramol di hydrochloride . Despite its name, it's not an opioid .
Indications
Opipramol is used for moody states associated with fear , restlessness , tension , sleep disorders or depression . It is also used for generalized anxiety disorder and somatoform disorders .
Opipramol should not have a negative impact on sleep quality.
Side effects
Side effects can include: tiredness, dizziness, gastrointestinal side effects such as nausea, sexual dysfunction such as B. Potency disorders. In high doses, the side effects of neuroleptics may occur, i.e. extrapyramidal (motor) disorders. In most cases, however, the side effects only occur at the beginning (first days to weeks) of taking and are only mildly pronounced. Opipramol should not be given with or 14 days after or before treatment with an MAOI . In addition, the consumption of alcoholic beverages should be avoided as this can lead to drowsiness .
Use during pregnancy and breastfeeding
There are insufficient case numbers for a well-founded risk assessment. Opipramol should only be prescribed during pregnancy if it is clearly indicated. Opipramol should not be used during breastfeeding because small amounts of the active ingredient are excreted in breast milk.
Withdrawal syndrome
Opipramol is not considered to be addictive, at least there is no known physical dependency potential from the active ingredient itself . The calming effect of opipramol can, however, be reversed on discontinuation and unmask any remaining disorders, so that the active ingredient - like all psychotropic drugs - should be slowly discontinued.
pharmacology
In contrast to most of the other representatives of this group, opipramol has no inhibitory effect on the uptake of biogenic amines (e.g. serotonin , noradrenaline ). Furthermore, opipramol has a structural similarity to the anti-epileptic carbamazepine , but opipramol has no anti-epileptic effects. The mode of action of many psychotropic drugs , including the opipramols, has not yet been fully clarified and is therefore still the subject of research. So is z. B. also unclear why the effect of structurally related substances can vary greatly. For this reason, the substance developed in Switzerland around 40 years ago is now primarily referred to as a "mood- enhancing anxiolytic " (as of 2013).
The following mechanisms of action have been proven:
- As a ligand / agonist, it primarily activates the σ1 receptor and also has a low affinity for the σ2 receptor . This property is held responsible for both antidepressant and anxiolytic effects of opipramol.
- It blocks with low affinity the serotonin receptor 5-HT 2A , which has been linked to anxiety , restlessness, panic, obsession and depression .
- It also blocks the dopamine receptor D 2 with low affinity . This receptor blockade is a typical property of so-called neuroleptics , which u. a. used in psychosis and schizophrenia . This multiple effect in the CNS explains the middle position of opipramol between classic antidepressants (classic ADs act on the serotonergic, noradrenergic and less often dopaminergic system by inhibiting the reuptake of the neurotransmitters at the (pre) synaptic gap or by modulating the neurotransmitter receptors on the neuron) and neuroleptics. The dopamine D 2 receptor is associated with anxiety, delusions , paranoid symptoms, nonsensical and compulsive actions, as well as pathological states of consciousness such as can occur in psychoses.
- In addition, histamine H 1 receptors are also blocked, but with a much lower affinity. This has a slightly sedating effect. Antihistamines of the older generation, which were mainly used against allergies and have now been replaced by newer antihistamines, had sedation and calming as an undesirable side effect. Today some old antihistamines are still for sale as sleeping pills (e.g. diphenhydramine )
- A very low anticholinergic effect, which is primarily responsible for the side effects (such as dry mouth)
The anxiolytic (anxiety-relieving, relaxing, calming) effect is probably due to the sum of the above-mentioned mechanisms of action.
When you start taking Opipramol preparations, a calming effect sets in quickly, only after about one to two weeks is this effect supplemented by a mood-enhancing active component. The sedative effect can already occur with the first ingestion.
The half-life of opipramol in the human body varies from person to person and is between 6 and 11 hours; the half-life does not increase with long-term use.
Trade names and manufacturers
Opipramol is sold under the trade name Insidon by Laboratoires Juvise Pharmaceuticals , there are several generic drugs , for example Opipram.
Individual evidence
- ↑ Entry on Opipramol. In: Römpp Online . Georg Thieme Verlag, accessed on July 7, 2014.
- ^ The Merck Index : An Encyclopedia of Chemicals, Drugs, and Biologicals . 14th Edition (Merck & Co., Inc.), Whitehouse Station, NJ, USA, 2006; P. 1181, ISBN 978-0-911910-00-1 .
- ↑ a b Data sheet Opipramol dihydrochloride from Sigma-Aldrich , accessed on April 16, 2011 ( PDF ).
- ^ A b c d e f g h A. Kleemann , J. Engel, B. Kutscher, D. Reichert: Pharmaceutical Substances - Synthesis, Patents, Applications , 4th edition (2000), Thieme-Verlag Stuttgart, ISBN 978-1 -58890-031-9 .
- ↑ Martin J. Lohse, Bruno Müller-Oerlinghausen: Psychopharmaka . In: U. Schwabe, D. Paffrath, W.-D. Ludwig, J. Klauber (Ed.): Drug Ordinance Report 2017 . Springer-Verlag GmbH, Berlin 2017, ISBN 978-3-662-54629-1 , p. 681-708 .
- ^ Specialist information of the Swiss Medicines Compendium: Insidon; Status: May 2000.
- ↑ Dirk Wolter, Walter Winkler: Approved indications and maximum dosages in psychiatry. In: Psychopharmakotherapie (2011), Volume 18, Issue 4, pp. 164–171.
- ↑ https://www.mediherz-shop.de/images/ecommerce/04/77/04775979_2010-07_de_o.pdf
- ↑ Press conference "Opipramol (Insidon): A classic with an innovative profile", July 18, 2005, Pharmacological Institute, Biozentrum of the University of Frankfurt ( Memento from January 20, 2016 in the Internet Archive ).
- ^ Schäfer, Spielmann, Vetter: Medicinal prescription in pregnancy and breastfeeding . Springer 7th edition 2006, ISBN 3-437-21332-6 .
- ↑ Müller, WE et al. (2004): Neuropharmacology of the anxiolytic drug opipramol, a sigma site ligand . In: Pharmacopsychiatry . Vol. 37, pp. 189-197. PMID 15547785 .
- ↑ Möller HJ, Volz HP, Reimann IW, Stoll KD: Opipramol for the treatment of generalized anxiety disorder: a placebo-controlled trial including an alprazolam-treated group . In: Journal of Clinical Psychopharmacology . 21, No. 1, February 2001, pp. 59-65. PMID 11199949 .
- ↑ Müller WE, Siebert B, Holoubek G, Gentsch C: Neuropharmacology of the anxiolytic drug opipramol, a sigma site ligand . In: Pharmacopsychiatry . 37 Suppl 3, November 2004, pp. S189-97. doi : 10.1055 / s-2004-832677 . PMID 15547785 .
- ^ Specialist information (SPC) from Holsten Pharma on Opipramol (PDF; 47.7 kB) ( Memento from March 4, 2016 in the Internet Archive ) , January 2006.
- ↑ Information for professionals (SPC) from Novartis Pharma on Opipramol (PDF; 42.1 kB).