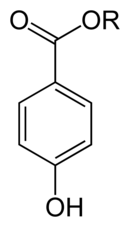
Parabens
Parabens is a collective name for 4-hydroxybenzoic acid ( paraben ) and its derivatives , such as salts and esters ( PHB esters for short ). The name is derived from para -hydroxy ben benzoic acid from. Like the related benzoic acid , they have an antimicrobial and fungicidal effect and the esters in particular are therefore often used as preservatives in the pharmaceutical industry, in cosmetics and in certain foods .
Natural occurrence
In nature, parabens and their derivatives occur in many plants such as cucumbers, carrots, onions, cherries, blueberries, cloudberries, currants, grapes, passion fruit or strawberries, in various spices but also in honey and royal jelly .
Representatives and characteristics
| Parabens | |||||||||
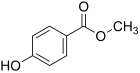
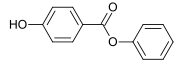
| Surname | Parabens | Methyl paraben | Ethyl paraben | Propyl paraben | Butyl paraben | Phenyl paraben | |||
| IUPAC name | 4-hydroxybenzoic acid | Methyl 4-hydroxybenzoate | Ethyl 4-hydroxybenzoate | Propyl 4-hydroxybenzoate | Butyl 4-hydroxybenzoate | Phenyl 4-hydroxybenzoate | |||
| Structural formula |

|
|

|

|

|
|
|||
| CAS number | 99-96-7 | 99-76-3 | 120-47-8 | 94-13-3 | 94-26-8 | 17696-62-7 | |||
| PubChem | 135 | 7456 | 8434 | 7175 | 7184 | 87250 | |||
| ECHA ID | 100.002.550 | 100.002.532 | 100,004,000 | 100.002.098 | 100.002.108 | 100,037,892 | |||
| Molecular formula | C 7 H 6 O 3 | C 8 H 8 O 3 | C 9 H 10 O 3 | C 10 H 12 O 3 | C 11 H 14 O 3 | C 13 H 10 O 3 | |||
| Molar mass ( g · mol -1 ) | 138.12 | 152.15 | 166.18 | 180.2 | 194.22 | 214.22 | |||
| Physical state | firmly | ||||||||
| Brief description | colorless, crystalline powder | ||||||||
| Melting point | 213–215 ° C (decomposition) | 125-128 ° C | 116-118 ° C | 95-98 ° C | 68-69 ° C | 182 ° C | |||
| boiling point | decomposition | 270–280 ° C (decomposition) | 297–298 ° C (decomposition) | from 310 ° C | |||||
| Solubility in water | 4.9 g l −1 at 20 ° C, 335 g l −1 at 100 ° C |
2.5 g l −1 at 20 ° C | 1.7 g l −1 at 20 ° C | 0.4 g l −1 at 20 ° C | 0.2 g l −1 at 20 ° C | ||||
|
GHS labeling |
danger |
- | - | - | - |
Caution |
|||
| H and P phrases | 318-335 | 412 | no H-phrases | no H-phrases | no H-phrases | 315-319 | |||
| 280-305 + 351 + 338 + 310 | 273 | no P-phrases | no P-phrases | no P-phrases | 302 + 352-305 + 351 + 338 | ||||
| Tox data | > 10000 mg kg −1 ( LD 50 , rat , oral ) | > 8000 mg kg −1 ( LD 50 , mouse , oral ) | 3000 mg kg −1 ( LD 50 , mouse , oral ) | 6332 mg kg −1 ( LD 50 , mouse , oral ) | 13200 mg kg −1 ( LD 50 , mouse , oral ) | ||||
use
Parabens are used as preservatives in various fields. In the technical area u. a. for the preservation of oils, fats, glues, shoe polishes.
drug
If necessary, parabens are used to preserve both medicinal products for external and internal use. In general, the use of preservatives requires special justification; it should be avoided whenever possible, especially when making preparations for children. The concentrations used must be as low as possible. When preservation is required, methyl and propyl 4-hydroxybenzoate are often used in the recipe as a fixed combination in the form of "Preserved Water DAC" ( Aqua conservans ). Because of its estrogen-like effect, propyl 4-hydroxybenzoate (propyl paraben) should be avoided in children and pregnant women. To use methyl and propyl parabens in human medicines that you take the published Committee for Medicinal Products (CHMP) 2015 Reflection Paper : Accordingly applies by the EFSA established for methyl and ethyl paraben and sodium salts thereof permitted daily dose ( acceptable daily intake, ADI ) of up to 10 mg / kg body weight also for drugs. The use of methyl paraben up to 0.2% as a preservative corresponds to this limit. Taking into account the effects of propylparaben on the female reproductive system found in animal studies, a maximum of 2 mg / kg body weight per day applies to the use of this substance in children and adults.
cosmetics
In cosmetics, parabens are used in creams, lotions, make-up, lipsticks, aftershave lotions, deodorants, soaps, sunscreens, depilatories and shampoos . The use in cosmetic products is regulated by Regulation (EC) No. 1223/2009 on cosmetic products (Cosmetics Regulation). Approved are 4-hydroxybenzoic acid ( paraben ), methyl paraben, ethyl paraben, propyl paraben, butyl paraben, and some of their sodium, potassium and calcium salts. There are maximum concentrations of 0.4% for methyl and ethyl paraben, 0.14% for propyl and butyl paraben and 0.8% for ester mixtures (each based on the acid content). Methyl paraben is predominantly used in cosmetics. Propyl and butyl paraben are used less frequently. Due to lack of data, the use of the less well-studied parabens is Isopropylparaben , Isobutylparaben , Phenylparaben , Benzylparaben and Pentylparaben banned since, 2014.
Food
Only methyl paraben and ethyl paraben and their sodium salts are permitted as additives in food . They are assigned the numbers E 214 (ethyl paraben), E 215 ( sodium ethyl paraben), E 218 (methyl paraben) and E 219 ( sodium methyl paraben ). The permitted uses are limited to a few applications (limit values each based on the free acid):
| Approved application | Limit value mg kg −1 or mg l −1 |
| Lab or Labaustauscher | 10,000 |
| Enzymes | 5000 |
| Egg paints | 4000 |
| Jelly coating for cooked, cured or dried meat products; Pies | 1000 |
| Cereal or potato based snacks, coated nuts | 300 |
| Confectionery, excluding chocolate | 300 |
Of the EFSA predetermined permitted daily dose ( English acceptable daily intake , ADI ) is for methylparaben and ethylparaben at 0 to 10 mg / kg body weight, twice as high as that for benzoic acid and salts thereof.
The use of E 216 (propyl paraben) and E 217 (sodium propyl paraben) in food has not been permitted in the EU since 2006.
Tobacco products
Until 2016, the Tobacco Ordinance allowed the use of E 214 (ethyl paraben), E 215 (sodium ethyl paraben), E 216 (propyl paraben) and E 217 (sodium propyl paraben) as preservatives for tobacco products , but not for cigars and not for cigarettes, with the exception of cigarette glue and tobacco foil approved. In the currently valid tobacco product regulation , the use is strictly prohibited by propyl paraben.
Health risks
The use of parabens has been linked to various undesirable side effects over the past few years. It should be noted here that parabens can enter the body not only through cosmetics, but also through medicines and foods.
Allergenic potential
Parabens are often criticized because they can trigger allergies . According to the Information Association of Dermatological Clinics (IVDK) 2011, however, “parabens rarely appear as the cause of contact sensitization on cosmetics”.
Cancer risk
British scientists (Darbre et al.) Reported in a 2004 publication that they were able to detect parabens in breast tumors. However, the authors did not conduct any studies on the paraben content in tumor-free tissues of the affected patients. However, later studies demonstrated systemic absorption by detecting intact paraben esters in human excretions. There was also no information on whether the patients had used paraben-containing deodorants at all before the tumors occurred. Despite these weaknesses in the study, warnings were given against the use of deodorants containing parabens, with reference to insufficient data. The warning was based on the fact that parabens have a structure similar to the hormone estrogen , which could possibly stimulate the cells of the breast tissue to grow uncontrollably. However, an exposure study found no association between antiperspirant or deodorant use and breast cancer. In addition, a distinction was made between different methods of armpit hair removal; here, too, no correlations were found. The Federal Institute for Risk Assessment (BfR) and the Scientific Committee for Consumer Safety (SCCS) of the European Commission also reviewed the work of Darbre et al. checked and see no association between paraben-based deodorant use and breast cancer.
Hormonal effectiveness
Parabens (namely propyl and butyl paraben) were suspected of belonging to the so-called endocrine disruptors , that is, of interfering with the action of hormones in living things. Endocrine disruptors can cause harmful effects such as reproductive disorders and the "feminization" of males in fish, birds, reptiles and mammals. According to a study commissioned by the Environment Directorate-General in 2009, in vitro receptor binding studies found that parabens have estrogenic and anti-androgenic properties. In animal experiments on adult rats, certain parabens were able to lower testosterone levels and sperm production, but the study results were inconsistent. In addition, studies with paraben mixtures have shown an estrogenic effect. However, the overall data situation is incomplete.
A study carried out by scientists at the Helmholtz Center for Environmental Research (UFZ) in Leipzig from 2006 (published in 2020) came to the conclusion that children of women who were pregnant with n- butyl or iso- butyl If you use cosmetics that have been preserved with paraben, you have a higher risk of becoming overweight later. Accordingly, girls in particular tended to be more overweight if an increased parabens concentration was detectable in the mother's organism during pregnancy. For methyl, ethyl and n -propyl paraben, however, no connection was found.
Application restrictions
In the opinion of the Scientific Committee for Consumer Safety (SCCS) of the EU Commission from 2011, a hormonal effect due to parabens in cosmetic products is not to be expected in humans if the usual conditions of use are observed. Based on the assessment of the SCCS, the Federal Institute for Risk Assessment (BfR) summarized the status of the assessment of the parabens in a statement in January 2011. Accordingly, methyl and ethyl parabens are to be regarded as safe in the permitted concentration range. The estrogenic potency is very low. For butyl and propyl parabens, a maximum concentration of 0.19% is suggested because of their higher estrogenic potency. Use is considered safe up to this concentration. The isopropyl-, isobutyl-, pentyl- and phenylparaben, which are rarely used anyway, should be avoided for the time being due to the incomplete data situation. In 2015, the maximum concentrations for butyl and propyl paraben were implemented through an amendment to the Cosmetics Ordinance; isopropyl, isobutyl, pentyl and phenyl paraben have since been banned in cosmetic preparations.
Propyl paraben and butyl paraben have been banned in baby products that are used for skin care in the diaper area, such as wound protection creams, since 2015. The ban applies to all "diaper cosmetics" for children under three years of age. The basis is an assessment of the preservatives by the SCCS, as existing skin irritations, such as a sore bottom, can increase the penetration of parabens in babies under six months of age into the baby's skin. The two parabens were banned in children's cosmetics in Denmark as early as 2011. The use of propyl paraben and butyl paraben in the specified concentrations is still considered safe for children over the age of three.
Environmental toxicity
In the South Sea state of Palau , the use and sale of sunscreens containing 10 different substances have been banned since January 2020 in order to protect coral reefs . These substances, which have a harmful effect on the environment, are the compounds oxybenzone , octynoxate , octocrylene and 4-methylbenzylidene camphor , which are used as UV protection , as well as the preservatives triclosan , phenoxyethanol and the methyl, ethyl, butyl alcohol, which act as endocrine disruptors. and benzyl paraben.
Web links
- Stiftung Warentest: Parabens as preservatives in cosmetics , as of August 19, 2013
- European Scientific Committee on Consumer Safety: Parabens used in cosmetics , "Factsheet" after revision adopted on October 10, 2011
Individual evidence
- ↑ a b Entry on 4-hydroxybenzoic acid. In: Römpp Online . Georg Thieme Verlag, accessed December 8, 2019.
- ↑ What Are Parabens? - Uses, Benefits, and Chemical Safety Facts. In: chemicalsafetyfacts.org. June 15, 2019, accessed December 10, 2019 .
- ↑ Paraben Information. In: cosmeticsinfo.org. 2016, accessed December 10, 2019 .
- ↑ Kaisu Määttä, Afaf Kamal-Eldin, Riitta Törrönen: Phenolic Compounds in Berries of Black, Red, Green, and White Currants (Ribes sp.) . In: Antioxidants & Redox Signaling . tape 3 , no. 6 , December 2001, p. 981 , doi : 10.1089 / 152308601317203521 .
- ↑ AY Badjah Hadj Ahmed, Munir S. Obbed, Saikh M. Wabaidur, Zeid A. AlOthman, Nora H. Al-Shaalan: High-Performance Liquid Chromatography Analysis of Phenolic Acid, flavonoid and phenolic content in Various Natural Yemeni Honeys Using Multi -walled Carbon Nanotubes as a Solid-Phase Extraction Adsorbent . In: Journal of Agricultural and Food Chemistry . tape 62 , no. 24 , June 5, 2014, pp. 5443 , doi : 10.1021 / jf5011758 .
- ↑ Hajimu Ishiwata, Yuiko Takeda, Takashi Yamada, Yoshinori Watanabe, Takeshi Hosagai, Sumio Ito, Hiroyuki Sakurai, Gaku Aoki, Nobuyuki Ushiama: Determination and confirmation of methyl ‐ hydroxybenzoate in royal jelly and other foods produced by the honey bee . In: Food Additives and Contaminants . tape 12 , no. 2 , March 1995, p. 281 , doi : 10.1080 / 02652039509374302 .
- ↑ Yoon-Han Kang, Charlotte C. Parker, Andrew C. Smith, Keith W. Waldron: Characterization and Distribution of Phenolics in Carrot Cell Walls . In: Journal of Agricultural and Food Chemistry . tape 56 , no. 18 , September 24, 2008, p. 8558 , doi : 10.1021 / jf801540k .
- ↑ Jennifer Smith-Becker, Eric Marois, Elisabeth J. Huguet, Sharon L. Midland, James J. Sims, Noel T. Keen: Accumulation of Salicylic Acid and 4-Hydroxybenzoic Acid in Phloem Fluids of Cucumber during Systemic Acquired Resistance Is Preceded by a Transient Increase in Phenylalanine Ammonia-Lyase Activity in Petioles and Stems . In: Plant Physiology . tape 116 , no. 1 , January 1, 1998, pp. 231 , doi : 10.1104 / pp.116.1.231 .
- ↑ George A. Burdock: Fenaroli's Handbook of Flavor Ingredients . CRC Press, 2001, ISBN 978-1-4398-6327-5 , pp. 1140 ( limited preview in Google Book search).
- ↑ a b c d e f Entry on 4-hydroxybenzoic acid in the GESTIS substance database of the IFA , accessed on December 8, 2019(JavaScript required) .
- ↑ a b c d e f Entry on methyl 4-hydroxybenzoate in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ a b c d e f Entry on ethyl 4-hydroxybenzoate in the GESTIS substance database of the IFA , accessed on December 1, 2007(JavaScript required) .
- ↑ a b Data sheet Propyl 4-hydroxybenzoate from Sigma-Aldrich , accessed on May 9, 2017 ( PDF ).
- ↑ a b c Entry for CAS no. 94-26-8 in the GESTIS substance database of the IFA , accessed on July 9, 2016(JavaScript required) .
- ↑ a b c d Datasheet Phenyl 4-hydroxybenzoate, analytical standard from Sigma-Aldrich , accessed on December 14, 2019 ( PDF ).
- ↑ a b c d e f Entry for CAS no. 94-13-3 in the GESTIS substance database of the IFA , accessed on July 9, 2016(JavaScript required) .
- ^ Entry on methylparaben in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on December 8, 2019.
- ↑ Entry on ethyl paraben in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on December 8, 2019.
- ↑ Entry on propyl paraben in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on December 8, 2019.
- ↑ Entry on butyl paraben in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on December 8, 2019.
- ↑ Alfred Fahr , Voigt Pharmazeutische Technologie , Deutscher Apothekerverlag, 12th edition (2010), ISBN 978-3-7692-6194-3 , Chapter 5 Basic and auxiliary materials in drug formulation, p. 192.
- ↑ KH Bauer, K.-H. Fromming, C. Führer: Pharmaceutical Technology . 2nd edition, Thieme Verlag (1989), p. 135.
- ↑ HA Schiffter Vintage Sign: protection against corruption - a preservative in the formulation , German Apotheker Zeitung, October 6, 2016th
- ↑ Guideline on Excipients in the Dossier for Application for Marketing Authorization of a Medicinal Product (PDF), Committee for Medicinal Products for Human Use of the European Medicines Agency, June 2007 (English).
- ^ Preserved water DAC , DAC / NRF recipe tips 2016, January 14, 2016.
- ↑ M. Haß: Formulation production - suspensions galenically complex , Pharmazeutische Zeitung, September 27, 2019.
- ↑ Reflection paper on the use of methyl- and propylparaben as excipients in human medicinal products for oral use (PDF), Committee on Medicinal Products for Human Use of the European Medicines Agency, October 2015 (English).
- ↑ Parabens as preservatives in cosmetics. Unnecessary uncertainty. Stiftung Warentest , August 19, 2013, accessed on December 9, 2019 .
- ↑ a b c REGULATION (EU) No. 358/2014 OF THE COMMISSION of April 9, 2014 (PDF; 334 kB), accessed on December 8, 2019
- ↑ a b c DIRECTIVE 2006/52 / EG (PDF; 110 kB), accessed on December 8, 2019
- ↑ Additive Admission Ordinance, Annex 5
- ↑ Tobacco Ordinance, Annex 1, repealed May 19, 2016
- ↑ Tobacco Products Ordinance, Appendix 1
- ↑ Stiftung Warentest: Medicines assessed . (December 1, 2012, chargeable).
- ↑ Lange-Ionescu, S. et al. (1996) Contact allergy in patients with congestive dermatitis or eczema of the legs. Dermatoses 44, 14-22.
- ^ Stiftung Warentest: Allergy to Parastoffe test.de December 1, 2012.
- ↑ GD Society for Dermopharmacy eV: contact allergies to cosmetics (PDF, 17 kB) press release of 4 April 2011th
- ↑ PD Darbre, A. Aljarrah, WR Miller, NG Coldham, MJ Sauer, GS Pope: Concentrations of parabens in human breast Tumors. In: Journal of applied toxicology: JAT. Volume 24, number 1, 2004 Jan-Feb, pp. 5-13, doi : 10.1002 / jat.958 . PMID 14745841 .
- ↑ Philippa D. Darbre, Philip W. Harvey: Paraben esters: review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks . In: Journal of Applied Toxicology . tape 28 , no. 5 , July 1, 2008, p. 561-578 , doi : 10.1002 / jat.1358 .
- ↑ krebshilfe.net - W. Parzefall, C. Drucker, N. Erlach, A. Losert, M. Micksche: Statement on a possible cancer risk from parabens ( Memento from January 6, 2014 in the Internet Archive ) (PDF file; 12 kB ) As of February 13, 2004.
- ↑ Bavarian State Office for Health and Food Safety: Breast cancer risk from deodorants containing parabens .
- ↑ BfR: Deodorants containing parabens and the development of breast cancer (February 13, 2004) (PDF; 64 kB).
- ↑ EU SCCS - Opinion on Parabens (2011) (PDF; 313 kB).
- ^ Opinion (PDF; 168 kB) of the Scientific Committee on Consumer Products on parabens, deodorants and breast cancer (as of January 2005).
- ↑ State of the Art of Endocrine Disruptors - Final Report , Report on the study commissioned by DG Environment in 2009 on the status of the assessment of endocrine disruptors, December 23, 2011.
- ↑ Maternal paraben exposure triggers childhood overweight development , publication by Leppert, B .; Strunz, S .; Seiwert, B .; Schlittenbauer, L .; Schlichting, R .; Pfeiffer, C .; Röder, S .; Bauer, M .; Borte, M .; Stangl, GI; Schöneberg, T .; Schulz, A .; Karkossa, I .; Rolle-Kampczyk, UE; Thürmann, L .; von Bergen, M .; Escher, BI; Boy, KM; Reemtsma, T .; Lehmann, I .; Polte, T. in Nature Communication, 2020
- ↑ Parabens promote obesity in children , Deutschlandfunk, 2020
- ↑ Opinion on Parabens, COLIPA n ° P82 (PDF) of the Scientific Committee on Consumer Safety on the safety of use of parabens in cosmetic products from December 14, 2010.
- ↑ BfR: Use of Parabens in Cosmetic Products (January 28, 2011) (PDF; 65 kB).
- ↑ a b REGULATION (EU) No. 1004/2014 OF THE COMMISSION of September 18, 2014 (PDF)
- ↑ Cosmetics: EU bans two parabens in baby cream, test.de from October 20, 2014, accessed on November 6, 2014.
- ^ The Republic of Palau Bans Sunscreen Chemicals to Protect its Coral Reefs and UNESCO World Heritage site - International Coral Reef Initiative. In: icriforum.org. Retrieved February 20, 2020 .