4-hydroxybenzoic acid methyl ester
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
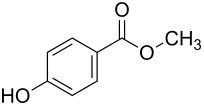
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 4-hydroxybenzoic acid methyl ester | |||||||||||||||
| other names | ||||||||||||||||
| Molecular formula | C 8 H 8 O 3 | |||||||||||||||
| Brief description |
white, almost odorless, crystalline powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 152.15 g · mol -1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.361 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
125-128 ° C |
|||||||||||||||
| boiling point |
Decomposes at 270-280 ° C |
|||||||||||||||
| Vapor pressure |
8.63 · 10 −2 Pa (50 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Methyl 4-hydroxybenzoate , and para -Hydroxybenzoesäuremethylester ( PHB-Methylester ), the Methylester of the aromatic carboxylic acid 4-hydroxybenzoic acid and one of the parabens . Nipagin is also used as a common name.
properties
4-Hydroxybenzoic acid methyl ester is a white, odorless, crystalline substance that has a slightly burning taste and has a weak anesthetic effect. The melting point is 126.0 ° C with a melting enthalpy of 166.5 J g −1 . The compound crystallizes in a monoclinic crystal lattice with the space group Cc . According to August, the vapor pressure function results according to ln (P) = −A / T + B (P in Pa, T in K) with A = 34.3 ± 0.3 and B = 11889 ± 92 in the temperature range from 30 ° C to 54 ° C. A molar enthalpy of sublimation of 98.8 kJ · mol −1 can be derived from the vapor pressure function . The compound dissolves little in water (2.5 g / l at 25 ° C.), but dissolves well in organic solvents such as acetone , chloroform , ethanol and ether . Phenolate (sodium methyl p-hydroxybenzoate) is formed in alkaline solutions .
Solubility in various solvents (at 25 ° C, in g / 100 g solvent) solvent water Water (80 ° C) Methanol Ethanol Propylene glycol acetone Diethyl ether peanut oil solubility 0.25 2.0 59 52 22nd 64 23 0.5
The methyl 4-hydroxybenzoate forms eutectically melting mixtures with other 4-hydroxybenzoic acid esters .
Eutectics for mixtures of 4-hydroxybenzoic acid esters Ethyl paraben Propyl paraben Butyl paraben Mole fraction of methyl paraben 0.46 0.35 0.78 Eutectic melting point 88.5 ° C 77.8 ° C 59.8 ° C
use
4-Hydroxybenzoic acid methyl ester is used as a preservative, for example in shampoos , shower gels and other cosmetics as well as in liquid medicines. It is also used in research to prevent undesirable fungal growth on bacteriological agar plates .
PHB methyl ester is approved in the EU as a food additive with the number E 218 . The sodium salt ( sodium methyl p- hydroxybenzoate , E 219 ) is usually used because of its better solubility .
Health risks
4-Hydroxybenzoic acid methyl ester - like parabens in general - can cause pseudo- allergic reactions in humans, especially in asthmatics , such as B. hives or asthmatic attacks.
4-Hydroxybenzoic acid methyl ester was included in the EU's ongoing action plan ( CoRAP ) in 2012 in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The causes of the uptake of methyl 4-hydroxybenzoate were concerns about its classification as a CMR substance, consumer use , exposure of sensitive population groups , high (aggregated) tonnage and widespread use, as well as a potential endocrine disruptor . The re-evaluation has been running since 2014 and is carried out by France . In order to be able to reach a final assessment, further information was requested.
Occurrence
In nature, the substance plays a role as a sex attractant of the provisional bitch . It is also a natural ingredient in royal jelly from bees .
Individual evidence
- ↑ Entry on METHYL PARABS in the CosIng database of the EU Commission, accessed on December 28, 2019.
- ↑ a b c d e f Entry on methyl 4-hydroxybenzoate in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ a b c d e Giordano, F .; Bettini, R .; Donini, C .; Gazzaniga, A .; Caira, MR; Zhang, GGZ; Grant, DJW: Physical properties of parabens and their mixtures: Solubility in water, thermal behavior, and crystal structures in J. Pharm. Sci. 88 (1999) 1210-1216, doi : 10.1021 / js9900452 .
- ↑ a b c Perlovich, GL; Rodionov, SV; Bauer-Brandl, A .: Thermodynamics of solubility, sublimation and solvation processes of parabens in Eur. J. Pharm. Sci. 24 (2005) 25-33, doi : 10.1016 / j.ejps.2004.09.007 .
- ↑ European Pharmacopoeia, 8th edition, Grundwerk 2014, p. 4078.
- ↑ Entry on 4-hydroxybenzoic acid methyl ester in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ^ Hermann PT Ammon, Curt Hunnius: Hunnius Pharmaceutical Dictionary , 2004, de Gruyter Verlag , ISBN 3-11-017475-8 .
- ↑ Entry on 4-hydroxybenzoates. In: Römpp Online . Georg Thieme Verlag, accessed on July 10, 2016.
- ↑ Markus DW Lipp: Emergency training for dentists: Prophylaxis, diagnosis, therapy , 1997, Schlütersche Verlagsgesellschaft , ISBN 3-87706-465-5 .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): Methyl 4-hydroxybenzoate , accessed on March 26, 2019.
- ↑ Goodwin, M. et al. : Sex pheromones in the dog. In: Science (1979) Vol. 203, pp. 559-561; PMID 569903 ; doi : 10.1126 / science.569903 .
- ↑ Ishiwata et al .: Determination and confirmation of methyl p-hydroxybenzoate in royal jelly and other foods produced by the honey bee. in: Food additives & contaminants (1995) Volume 12 (2), pp. 281-285; PMID 7781824 .