Pelargonic acid methyl ester
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
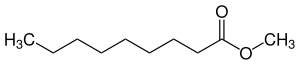
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Pelargonic acid methyl ester | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 10 H 20 O 2 | |||||||||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 172.27 g mol −1 | |||||||||||||||||||||
| Physical state |
liquid |
|||||||||||||||||||||
| density |
0.875 g cm −3 |
|||||||||||||||||||||
| Melting point |
−30 ° C |
|||||||||||||||||||||
| boiling point |
213 ° C |
|||||||||||||||||||||
| Vapor pressure |
0.1 hPa (20 ° C) |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| Refractive index |
1.421 (20 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
Pelargonic acid methyl ester is a fruit flavor and a chemical compound from the group of carboxylic acid esters .
Occurrence
Pelargonic acid methyl ester was found in Orris derivatives, apples , bananas , blackberries , baked potatoes, blue cheese , beef fat, hop oil , white wine , star fruit , prickly pears , bourbon vanilla, mountain papaya, monkey orange and rooibos tea ( Aspalathus linearis ).
Extraction and presentation
Pelargonic acid methyl ester can be obtained industrially by acid-catalyzed esterification of pelargonic acid with methanol at temperatures of 30-60 ° C in the presence of acidic cation exchangers based on a sulfonated styrene-divinylbenzene copolymer and subsequent rectification .
Another possibility is the catalytic hydrogenation of 1,5-octadienecarboxylic acid methyl ester using palladium (II) chloride in methanol as solvent.
properties
Pelargonic acid methyl ester is a flammable, difficult to ignite, colorless liquid that is practically insoluble in water. The compound has a wine-like and coconut-like odor. Below 10 ppm it has a sweet, coconut-like taste.
use
Pelargonic acid methyl ester for the synthesis of vanillyl nonanoate , a model compound of capsinoids , and used as a flavoring substance .
safety instructions
The vapors of pelargonic acid methyl ester can form an explosive mixture with air ( flash point 87 ° C).
Individual evidence
- ↑ Entry on METHYL PELARGONATE in the CosIng database of the EU Commission, accessed on July 6, 2020.
- ↑ a b c d e f g h i j k Entry on pelargonic acid methyl ester in the GESTIS substance database of the IFA , accessed on December 14, 2018(JavaScript required) .
- ↑ a b c d e George A. Burdock: Fenaroli's Handbook of Flavor Ingredients . CRC Press, 2004, ISBN 978-1-4200-3787-6 , pp. 1240 ( limited preview in Google Book search).
- ↑ a b Data sheet Methyl nonanoate, 98% from Sigma-Aldrich , accessed on December 14, 2018 ( PDF ).
- ↑ Mamta Sharma, Ravinder Kumar Wanchoo, Amrit Pal Toor: Adsorption and Kinetic Parameters for Synthesis of Methyl Nonanoate over Heterogeneous Catalysts. In: Ind. Eng. Chem. Res. 2012, 51, 44, 14367-14375, ACS Publications , October 2, 2012, doi : 10.1021 / ie301661n .




