Phosphorus trichloride
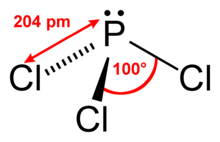
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Phosphorus trichloride | ||||||||||||||||||
| other names |
Phosphorus (III) chloride |
||||||||||||||||||
| Molecular formula | PCl 3 | ||||||||||||||||||
| Brief description |
colorless liquid with a pungent odor that smokes in humid air |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 137.33 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.57 g cm −3 (21 ° C) |
||||||||||||||||||
| Melting point |
−93.6 ° C |
||||||||||||||||||
| boiling point |
76 ° C |
||||||||||||||||||
| Vapor pressure |
133.3 hPa (21 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.5122 (21 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
|
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Phosphorus trichloride or phosphorus (III) chloride is a colorless, poisonous and strongly corrosive liquid with a pungent odor that smokes in moist air as a result of hydrolysis .
Manufacturing
The direct reaction of chlorine with white phosphorus , P 4 , results in small amounts of phosphorus pentachloride , PCl 5 . The latter also reacts with excess phosphorus to form phosphorus trichloride.
properties
Phosphorus trichloride is the acid chloride of phosphorous acid , a dyadic tautomer of phosphonic acid . As a result, hydrolysis to this and hydrogen chloride takes place with water :
Hydrolysis of the phosphorus (III) chloride to phosphorous acid / phosphonic acid
Phosphorous acid esters are formed in an analogous manner with alcohols . The chlorine atoms are gradually substituted:
Conversion of phosphorus trichloride with methanol - gradual substitution
The reactions with amines , thiols or ( pseudo- ) halides take place analogously .
Phosphorus trichloride is a very strong reducing agent and is used as an oxygen acceptor. If it functions as the latter, phosphoryl chloride is formed as an oxidation product , which accompanies phosphorus chloride as an impurity when it comes into contact with air.
Phosphorus trichloride can also be chlorinated to phosphorus pentachloride:
use
Phosphorus trichloride is a raw material in the chemical industry that is used in a variety of ways. It is needed for the production of other phosphorus derivatives such as insecticides, pharmaceuticals, di- and trialkyl phosphites, phosphoryl chloride, thiophosphoryl chloride and the like. v. a. m.
Carboxylic acid chlorides can be produced on a laboratory scale with the aid of phosphorus trichloride , a method which, however, does not have any advantages compared to the production with thionyl chloride or oxalyl chloride .
Individual evidence
- ↑ a b c d e entry on phosphorus chlorides. In: Römpp Online . Georg Thieme Verlag, accessed on March 3, 2014.
- ↑ a b c d e f g h Entry on phosphorus trichloride in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Index of Refraction of Inorganic Liquids, pp. 4-140.
- ↑ Entry on Phosphorus trichloride in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 7719-12-2 or phosphorus trichloride ), accessed on September 14, 2019.
- ↑ Limit values for working materials ( Memento of the original from September 23, 2015 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. , Ordinance of the Federal Minister for Labor, Social Affairs and Consumer Protection (Limit Values Ordinance GKV 2011), Austria.
- ^ A b Karl A. Hofmann: Inorganic Chemistry . Springer-Verlag, 2013, ISBN 978-3-663-14240-9 , pp. 273 ( limited preview in Google Book search).
- ^ Egon Wiberg: Textbook of Inorganic Chemistry - With an appendix: History of chemistry . Walter de Gruyter, 2011, ISBN 978-3-11-023832-7 , p. 260 ( limited preview in Google Book Search).








![{\ mathrm {2 \ PCl_ {3} +2 \ Cl_ {2} \ rightarrow 2 \ PCl_ {5} \ \ rightleftharpoons \ [PCl_ {4}] ^ {+} [PCl_ {6}] ^ {-}} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/5c776207ef6d989dc8ab38eee3200b74ebff5602)