Prion
Prions are proteins that can exist in the animal organism in both physiological (normal) and pathogenic (health-damaging) conformations (structures). They do not multiply through division, but through induced changes in neighboring molecules.
The English name prion was proposed in 1982 by Stanley Prusiner , who received the Nobel Prize in 1997 for the discovery of prions . It is a portmanteau derived from the words pr otein and infect ion and refers to the ability of prions to transfer their conformation to other prions. So it is not about living beings, but about organic toxins (poisons) with infectious properties.
The body's own prions are increasingly found in brain tissue, so that pathological changes can have serious consequences for the organism. The pathogenic prions are very likely to be responsible for Creutzfeldt-Jakob disease in humans, BSE (mad cow disease) in cattle, or scrapie in sheep . They are most likely to enter the body through contaminated food (e.g. in BSE , chronic wasting disease or kuru ). Other routes of infection such as smear infection could not yet be ruled out. Pathogenic prions can also arise from the spontaneous refolding of endogenous prions (e.g. familial variant of Creutzfeldt-Jakob disease, familial insomnia ).
Even in today's modern medicine, curative treatment of prion diseases is not possible, so that measures are only possible in the context of palliative medicine . All diseases also have in common that they lead to a spongiform (spongy) disintegration of the brain or the vegetative nervous system and are therefore fundamentally fatal. The pathogenic prevalence of diseases caused by prions is generally considered to be extremely low.
Basically, pathogenic prions must be differentiated from other pathogens such as viruses , bacteria or fungi , as they do not contain any DNA or RNA . Not only are they of great scientific and medical interest, but the "BSE crisis" also had a major impact on areas such as agriculture , consumer protection and politics .
The simplified prion hypothesis and peculiarities of prion diseases
One of the many occurring in the animal body protein is PrP C ( Pr ion P rotein c ellular = cellular prion protein). It is found primarily in the nervous system , especially in the brain . The prions differ more or less slightly between the various animal species and possibly also within one animal species. PrP C occurs mainly on the cell surface and protects the cells from divalent copper ions, H 2 O 2 and free radicals . Furthermore, it is assumed that it is one of the first sensors in the cellular defense against reactive oxygen and free radicals and has an impact on the enzymatic breakdown of free radicals.
Apparatus of this normal protein PrP C into contact with a PrP Sc said protein ( Pr ion P rotein Sc therapy; pathogenic form of the prion protein in the form of first at at scrapie was found diseased animals), PrP takes C in the form of PrP Sc on, “it flips over”, it changes its conformation. A chain reaction develops in which more and more PrP C are converted into PrP Sc . Large amounts of PrP Sc are destructive to the brain because they are insoluble and are deposited in the cells. As a result, these cells die; holes appear in the brain and a spongy structure is created. Hence the name of this disease: spongiform encephalopathy, spongy brain disease. Prion diseases are always fatal.
The initiation of the disease can take place in three ways, which is unique among all diseases:
- sporadically, d. H. by chance or without any recognizable cause: PrP C “randomly” folds into PrP Sc and thus triggers the chain reaction. One example is the classic form of Creutzfeldt-Jakob disease (sCJD).
- genetic, d. H. due to a “mistake” in the genetic material: the PRNP gene, on which the information for the production of PrP C is stored in the form of DNA , can contain a mutation. The then altered protein is more prone to converting to PrP Sc . The mutation can be passed on from parents to children. Examples are the familial form of Creutzfeldt-Jakob disease (fCJD), Gerstmann-Sträussler-Scheinker syndrome (GSS) and fatal familial insomnia (FFI Fatal Familial Insomnia).
- through transmission or “contagion”: If you bring yourself to PrP Sc from outside , your own PrP C can in turn be converted into PrP Sc , but not under all circumstances. It depends on what amount is supplied, in what way and what type of PrP Sc it is exactly. Infection in everyday contact with patients is not possible. On the other hand, a transfer is quite possible if material containing a lot of PrP Sc, i.e. e.g. B. brain of sick animals or humans, gets into the blood or in the worst case directly into the brain. This is the case, for example, with the iatrogenic form of Creutzfeldt-Jakob disease (iCJD). During operations on the brain, inadequately sterilized instruments accidentally brought prions from sick people into the brains of healthy people. Another example is Kuru , a disease in Papua New Guinea ; Members of the affected tribe took in the brains of the deceased and thus high amounts of PrP Sc as part of cultural rites . The most prominent example is certainly BSE ("mad cow disease"). According to the theory, which is largely recognized in science, these PrP Sc were spread by animal meal , which u. a. made from carcasses of sheep infected with scrapie and then fed to cattle. In addition, the UK had changed the process of making meat-and-bone meal (lower temperature or pressure) so that these PrP Sc could survive the manufacturing process. To a lesser extent, other animals (cats, zoo animals) have also been infected by this animal meal or by feeding parts from sick cattle. Finally, especially in Great Britain , people fell ill with a new form of Creutzfeldt-Jakob disease, the "new variant" (nvCJD or vCJD), probably due to the consumption of meat or brain or spinal cord tissue from BSE cows. In experiments with mice, Swiss researchers discovered a new route of infection, via the air we breathe. Whether such an infection is also possible in humans is still controversial.
Familial forms of prion diseases can also be transferred in experiments; for example, PrP Sc , which arises in humans due to genetic disposition, can trigger the disease in mice if it has been injected into the brain beforehand.
Prions are very resistant to common disinfection and sterilization processes , which was one of the reasons for the iCJD cases and the BSE crisis. Today there are strict regulations tailored to the difficult inactivation of prions for the sterilization of material that has come into contact with tissue that may contain prion.
The prion hypothesis is now considered to be relatively secure. However, the fact that another factor plays a role besides the PrP Sc cannot yet be definitively ruled out. After the intensive search for viruses, viroids or nucleic acids was unsuccessful at all, there are hardly any scientists who continue to pursue this path. Outsider opinions occasionally circulate in public, such as the organophosphate theory , according to which BSE is related to insecticides, but for which there is no scientific evidence.
History of prion research
Individual prion diseases were described a long time ago ( scrapie , the prion disease of the sheep, 1759 by Johann George Leopoldt ; CJD 1920 by Hans Gerhard Creutzfeldt ) without knowing anything about the cause of these diseases or being able to classify them into a group. After the transferability of scrapie was proven in 1932 and Kuru was first described in 1957 , the similarity of these diseases was established by William J. Hadlow in the late 1950s and Kuru was also experimentally transferred to monkeys. In 1966, Tikvah Alper and co-workers discovered that the pathogen was too small to be a virus and apparently did not contain any nucleic acid. This led to a "protein only" hypothesis for the pathogen, although it remained unclear how such a protein could multiply. Many therefore assumed that lentiviruses were the most likely cause.
The "prion hypothesis" published in 1982 by Stanley Prusiner , who followed the work of Daniel C. Gajdusek , who had already unconsciously discovered a pathogenic prion, was initially received critically in science, as a nucleic acid-free infectious agent was previously inconceivable. In retrospect, however, this hypothesis turned out to be groundbreaking and in 1997 Prusiner was honored with the Nobel Prize for his work in the field of prion research. In the years after the establishment of this hypothesis, indications for the correctness of this hypothesis could be obtained in numerous experiments, but no definitive proof. The BSE epidemic began in Great Britain in 1986 and the first cases of vCJD were described in 1996.
The proof that recombinant prion protein can cause diseases (which fulfills Koch's postulate ) was achieved in 2010.
Politicians tried to make up for their failures in prevention and consumer protection by, among other things, generous spending on prion research. Numerous working groups were set up, centers built and associations founded. Prion research has been intensified and accelerated.
In 2007, doubts arose as to whether the content of pathogenic prions in a tissue correlates in every case with its infectivity .
New research results from the American researcher Susan Lindquist show that prions play an important role in neurogenesis (the development of new nerve cells in the brain).
Structure of the prion protein
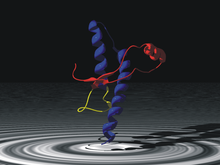
43% of the physiological (i.e. normal or non-pathogenic) prions have the structure of alpha helices . The pathogenic forms consist of only 30% alpha-helices , 43% consist of beta-sheet structures. The danger of pathogenic prions is that they are able to convert physiological, non-pathogenic prions into pathogenic ones.
The prion protein is a human glycoprotein consisting of 253 amino acids (AA), which is encoded in the prion protein gene (PRNP). The AA homology to other mammals is 85% or more; between cattle and humans there are e.g. B. 13 AS differences. One or more mutations are known that lead to fCJD, GSS or FFI. At codon 129 there is a methionine / valine polymorphism , which is one of the decisive factors for the onset and course of the disease. PrP C contains a large proportion of alpha helices, PrP Sc more beta-sheet structures, but both contain the same primary amino acid sequence .
The exact process of “folding” from PrP C to PrP Sc is still unknown. This changes the properties of the prion protein; the PrP Sc is less water-soluble because the hydrophobic chains do not point to the inside of the protein tertiary structure , as is usual with the α-helix . In addition, PrP Sc is largely resistant to many disinfectants, ionizing and UV radiation and is heat-stable. Moist heat (131 ° C) in autoclaves used for sterilization in medicine only destroys the PrP Sc after two hours, so that medical instruments have to be autoclaved four times in a row; in dry heat, the prion is only inactivated after 60 minutes at 200 ° C difficult to digest due to proteases (proteases can best “cut up” a protein when it is unfolded, but denaturation in the body is less possible due to the changed secondary structure). PrP C is mainly localized at synapses .
According to the latest findings, PrP C play a role in the formation of blood-forming stem cells (see below). Prion protein knockout mice show slower recovery after a stroke, and the mice are also prone to obesity . However, there is evidence of a role as a copper-binding protein at the synapse. According to a publication by US researchers in the journal PNAS , normal prion proteins maintain the regenerative capacity of blood-forming stem cells. In these cells, prions appear in the cell membrane and apparently do not perform any important tasks - at least as long as the body is healthy.
Pathology and symptoms of prion diseases
Prion diseases are primarily characterized by motor disorders such as ataxia and - most noticeably in humans - cognitive problems up to dementia . After an incubation period of years to decades, the diseases are always fatal. The neuropathological examination under the light microscope reveals spongy (spongy) changes in the brain and, depending on the disease, differently pronounced deposits such as amyloids , Kuru plaques and florid plaques.
Selection of current research areas
- Function of the PrP C
- exact structure of PrP C and PrP Sc
- Transmission routes
- Blood (rapid) tests
- Therapy options
- Opportunities for prevention, risk assessment and monitoring
It is assumed that the prion disease is infected through prion-associated proteins and not through the actual prion protein. Research into prion-associated proteins is therefore of particular interest.
Reporting requirement
In Switzerland, the positive laboratory analysis findings to "prion" is for laboratories reporting requirement and that after the Epidemics Act (EpG) in connection with the epidemic Regulation and Annex 3 of the Regulation of EDI on the reporting of observations of communicable diseases of man . This is understood as «positive evidence of PrPSc in clinical material (especially brain)».
literature
- Beat Hörnlimann, D. Riesner, H. Kretzschmar: Prions and prion diseases . de Gruyter, Berlin / New York 2001, ISBN 3-11-016361-6 .
- Martin H. Groschup: Prion diseases - diagnosis and pathogenesis. Springer, Vienna 2000, ISBN 3-211-83530-X .
- Claudio Soto: Prions - the new biology of proteins. CRC, Taylor & Francis, Boca Raton 2006, ISBN 0-8493-1442-9 .
- Sylvain Lehmann: Techniques in prion research. Birkhäuser, Basel 2004, ISBN 3-7643-2415-5 .
- Andrew F. Hill: Prion protein protocols. Humana Press, Totowa 2008, ISBN 978-1-58829-897-3 .
Web links
- Website National Reference Center for Prion Diseases, Georg-August University Göttingen
- Ingrid Schütt-Abraham, Roland Heynkes: Theoretical TSE research . Roland Heynkes website
- prionforschung.de - Prion Research · TSE Coordination Office Lower Saxony
- Stefanie Offermann: Blood test for prions: Researchers develop new diagnostic methods for the protein pathogens. In: Wissenschaft.de . August 29, 2005, archived from the original on February 23, 2006 (reference to Claudio Soto, Joaquín Castilla, Paula Saá: Detection of prions in blood . In: Nature Medicine 11/2005, August 28, 2005, pp. 982–985, doi : 10.1038 / nm1286 ).
- Katharina Schöbi: Prions on winding paths: Researcher: BSE pathogens could originally come from humans. In: Wissenschaft.de. September 2, 2005, archived from the original on November 8, 2005 (reference to the article by Alan Colchester and Nancy Colchester in: The Lancet , Vol. 366, pp. 856, 2005).
- Ilka Lehnen-Beyel: How prions travel from sheep to sheep: Study shows that the infectious proteins can be transmitted through the urine. In: Wissenschaft.de. October 14, 2005, archived from the original on December 25, 2005 (reference to the article by Harald Seeger et al. In: Science , Vol. 310, p. 324, 2005).
- Ilka Lehnen-Beyel: The Prions' Long Sleep : The incubation period can be more than fifty years. In: Wissenschaft.de. June 23, 2006, archived from the original on July 3, 2006 (reference to the article by John Collinge et al in: The Lancet , vol. 367, p. 2068).
- David S. Goodsell: Molecule of the Month: Prions. In: Protein Data Bank . May 2008, archived from the original on May 11, 2008 ; accessed on April 23, 2018 (English).
Individual evidence
- ^ Stanley B. Prusiner: Novel proteinaceous infectious particles cause scrapie . Science 1982, 216 (4542), pp. 136-144. PMID 6801762 .
- ↑ The word prion is also shown as an abbreviation for the term pr oteinaceous i nfectious particle used by Prusiner , cf. proteinaceous in the LEO dictionary
- ↑ See Reactive Oxygen Species-mediated β-Cleavage of the Prion Protein in the Cellular Response to Oxidative Stress. In: Journal of Biological Chemistry . Vol. 280, NO.43, pp. 35914-35921.
- ↑ See Haybaeck et al. Aerosols Transmit Prions to Immunocompetent and Immunodeficient Mice. In: PLoS Pathog . 7 (1), p. E1001257, 2011. doi: 10.1371 / journal.ppat.1001257
- ↑ Tikvah Alper, DA Haig, MC Clarke: The exceptionally small size of the scrapie agent . In: Biochemical and Biophysical Research Communications . tape 22 , no. 3 , p. 278–284 , doi : 10.1016 / 0006-291x (66) 90478-5 ( elsevier.com [accessed October 31, 2017]).
- ↑ Tikvah Alper, WA Cramp, DA Haig, MC Clarke: Does the Agent of Scrapie Replicate without Nucleic Acid? In: Nature . tape 214 , no. 5090 , May 20, 1967, p. 764–766 , doi : 10.1038 / 214764a0 ( nature.com [accessed October 31, 2017]).
- ^ R. Latarjet, B. Muel, DA Haig, MC Clarke, Tikvah Alper: Inactivation of the Scrapie Agent by Near Monochromatic Ultraviolet Light . In: Nature . tape 227 , no. 5265 , September 26, 1970, pp. 1341–1343 , doi : 10.1038 / 2271341a0 ( nature.com [accessed October 31, 2017]).
- ↑ Gisela Baumgart: Prusiner, Stanely Ben. In: Werner E. Gerabek , Bernhard D. Haage, Gundolf Keil , Wolfgang Wegner (eds.): Enzyklopädie Medizingeschichte. De Gruyter, Berlin / New York 2005, ISBN 3-11-015714-4 , p. 1188.
- ↑ Fei Wang, Xinhe Wang, Chong-Gang Yuan, Jiyan Ma: Generating a Prion with Bacterially Expressed Recombinant Prion Protein . In: Science . tape 327 , no. 5969 , February 26, 2010, ISSN 0036-8075 , p. 1132–1135 , doi : 10.1126 / science.1183748 , PMID 20110469 ( sciencemag.org [accessed October 31, 2017]).
- ^ Stephan Schröder-Köhne: Advances in prion research. April 21, 2005.
- ↑ RM Barron et al: High titres of TSE infectivity associated with extremely low levels of PrPSc in vivo. In: J Biol Chem . 2007 Oct 8. PMID 17923484
- ↑ P. Piccardo et al .: Accumulation of prion protein in the brain that is not associated with transmissible disease. In: Proc Natl Acad Sci USA . 2007 Mar 13; 104 (11), pp. 4712-4717. PMID 17360589 .
- ↑ Protein Data Bank: Human Prion Protein Fragment 121-230
- ↑ Notifiable communicable diseases and pathogens. (PDF, 4 MB) Guideline on mandatory notification 2020. Federal Office of Public Health FOPH, Communicable Diseases Department, February 23, 2020, p. 18 , accessed on March 8, 2020 .