Bovine spongiform encephalopathy
Bovine spongiform encephalopathy , BSE for short (German: "spongy regression of brain matter occurring in cattle"), also known colloquially as mad cow disease , is an animal disease . The fatal brain disease , especially in domestic cattle , is believed to be caused by prions (atypically folded proteins ).
General
Prion proteins are characteristic of the disease. These are abnormal proteins (PrP Sc ) that arise in the affected brain by refolding from the normal form of the protein (PrP C ). This triggers a disastrous biochemical process that leads to the deposition of the folded protein and, as a result, to the degeneration of the brain tissue. As the disease progresses, the affected brain takes on a sponge-like, perforated structure with thread-like, protein-containing deposits.
BSE belongs to the group of transmissible spongy encephalopathies (TSE) . It is believed that the new variant of the fatal Creutzfeldt-Jakob disease (now known as nvCJD) in humans is caused by the consumption of BSE-contaminated beef.
Another suspicion is that this disease appeared as early as the 19th century, but then disappeared again at the beginning of the 20th century. References to this can be found in books on cattle diseases from the end of the 19th century and in reports from the beginning of the 20th century. After 20 years of compulsory examinations of dead cattle and slaughter cattle of certain age groups, there are still isolated positive BSE findings without a clinic. These are z. Currently referred to as sporadic forms of BSE, which are associated with genetic characteristics. It can be assumed that like TSE in humans, it also occurs spontaneously in cattle at a very low frequency such as 1: 1 million or less.
transmission
Based on epidemiological studies, the consumption of infectious feed is now assumed to be the cause of BSE. In Great Britain, in the 1970s, the processing of animal carcasses in the rendering plants (TBA) was allowed at lower temperatures. As a result, only vegetative germs such as salmonella could be effectively killed. Originally, high-pressure sterilization was carried out at 133 ° C for 20 minutes to ensure the destruction of anthrax spores . The change in the temperature regime in the UK TCA is seen as essential for the start of the BSE crisis in the UK. At first one saw a relationship with scrapie ; Meanwhile, the prevailing view is that the previously unknown spontaneous form of BSE caused the BSE crisis due to insufficiently heated meat and bone meal in concentrate.
In Germany today the use of meat and bone meal from ruminants in cattle feed is completely prohibited. The high pressure sterilization of animal waste in the TBA was maintained in Germany. As with the other transmissible spongiform encephalopathies (including transmissible spongiform encephalopathies , TSE , such as Kuru , Chronic Wasting Disease ), it is transmitted through the consumption of infected animal products. Horizontal transmission from cow to cow is considered unlikely. The variants of PrP Sc which occur in cows (as in scrapie in sheep) probably migrate from the intestine to the central nervous system via nerve tracts . Scrapie in sheep differs from BSE in an additional way of spreading via the lymphatic system and has genetic components (strongly represented in the Suffolk breed, among others) that have led to a long-established natural occurrence.
Not all PrP Sc variants are pathogenic . The correlation between PrP Sc , amyloid deposits and spongiform encephalopathy was initially questioned, the connection is now considered to be certain. Prions are the only previously known infectious agents that do not contain nucleic acids.
Around five to ten percent of the calves of cows suffering from BSE also develop BSE. Similar to scrapie, vertical transmission of the disease from cow to calf through blood or amniotic fluid at birth is suspected. A transmission through a calf substitute feed containing PrP Sc instead of cow's milk is also possible. Pathogenic prions have so far not been found in milk, semen or embryo. Since wild deer and elk also develop transmissible spongiform encephalopathies ( Chronic Wasting Disease , CWD) without ingesting animal meal or other animal products (except breast milk), vertical infection routes (from cow to calf) are considered possible. However, the CWD prion is also demonstrably horizontally transferable; a transmission via aerosols could be proven in animal experiments with mice.
The denaturation of the PrP Sc by thermal disinfection processes only takes place at relatively high temperatures and pressures; under milder conditions, infectious PrP Sc particles can still be detected. These milder conditions were introduced in the UK and have led to the spread of BSE after feeding high protein concentrate mixes to cattle on dairy farms.
Course of the disease / symptoms
With the disease, the brain is perforated like a sponge and its functions are disturbed. The above-mentioned prions can be detected in tissue samples .
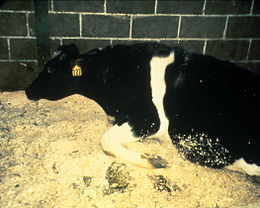
The affected animals show behavior changes and movement disorders. The cattle begin to stumble, trip over their own legs and react frightfully to noise and light stimuli. Muscle tremors, tongue play, and occasionally brisk ear play and phantom itching are observed.
Infected cattle usually develop BSE after an incubation period of four to five years and then die within a few months. As soon as clinical disturbances have occurred, the typical histological changes are regularly determined post-mortem.
Evidence / diagnosis
The diagnosis can be confirmed by a detailed examination of a special part of the brain (the medulla oblongata at the level of the obex ) of a dead animal.
The methods can be divided into:
- Histology : evidence of vacuoles (cavities)
- Immunohistochemistry : detection of the prions
- since 1999: rapid tests (known as BSE test ) ( ELISA , Western Blot , SDD-AGE ): antibodies to PrP Sc (see prion )
- since 2005: Protein Misfolding Cyclic Amplification
In the Western blot rapid test, tissue samples are taken from the obex region of the brain and used in duplicate with a control sample from a healthy cattle. The enzyme proteinase K , which breaks down the healthy PrP C , but not the modified PrP Sc , is added to the samples . The control sample remains untreated. Now all samples are analyzed by Western blot . A specific luminescent conjugated antibody against PrP C and PrP Sc is used here. In the case of negative samples, no band appears on the blot because the healthy protein PrP C has been degraded. In positive samples, the modified PrP Sc protein is displayed on negative film exposed through the gel. The control sample is used only to check all reactions and represents the natural PrP. The bands of the positive PrP Sc and the natural PrP C also differ slightly in terms of their position on the negative film.
This procedure was extremely time consuming and labor intensive in mass operation and was replaced by later developed applications.
Both the prions and the vacuoles can only be detected in the late stages of the disease, so the European Union has decided to only have cattle examined from 24 to 30 months (depending on the examination category). On January 1, 2009, the age limit for healthy slaughtered animals in Germany was raised to 48 months.
For a long time it was not possible to detect prions in living animals. In 2005, a test was presented with a clinical study that for the first time can also be reliably applied to living animals. To date, however, this test has not been approved.
Spread
The greatest incidence of BSE was recorded in 1992 with over 37,000 cases worldwide; very far predominantly in the UK. According to the United Nations, the number of BSE cases has steadily decreased since 2003. While there were still 1646 cases worldwide in 2003, 878 cases were recorded in 2004 and only 474 in 2005. In 2011, only 29 cases were reported , according to the USDA .
After the development of the rapid tests in 2000, the phase of active surveillance began in the European Union (EU) and in many member states only then were cases of BSE officially confirmed. The prior passive surveillance is based solely on the obligation to report people who handle cattle and has proven to be insufficient. In 2006, 320 BSE cases were registered in the EU, compared to 175 in 2007.
Germany
| Confirmed BSE cases in Germany since 2000 | ||||
|---|---|---|---|---|
| 2000 | 7th | |||
| 2001 | 125 | |||
| 2002 | 106 | |||
| 2003 | 54 | |||
| 2004 | 65 | |||
| 2005 | 32 | |||
| 2006 | 16 | |||
| 2007 | 4th | |||
| 2008 | 2 | |||
| 2009 | 2 | |||
| 2010 | 0 | |||
| 2011 | 0 | |||
| 2012 | 0 | |||
| 2013 | 0 | |||
| 2014 | 2 | |||
| 2015 | 0 | |||
| 2016 | 0 | |||
| 2017 | 0 | |||
| Source: BMEL, previously BMELV | ||||
After the first appearance of symptoms in cattle in the mid-1980s indicating a possible new disease, months passed before the similarity of the histological changes to scrapie in sheep suggested TSE. While strong efforts have been made in the UK to combat the disease, politicians and business circles have argued that the risk of it occurring in Germany has been greatly downplayed. The following events can be classified against this background:
The first suspected cases of BSE were registered in the Segeberg district as early as the early 1990s . However, the cases, which had risen to 24 by 1994, were not examined in detail (see the following paragraph); the carcasses were released for further processing. When the meat hygiene veterinarian Margrit Herbst, who was then in office there, went public with her findings in 1994 , her employer dismissed her without notice and - initially successfully - sued for not being allowed to continue spreading the relevant facts. The judgment was later overturned in full through the courts. She was awarded the Whistleblower Prize in 2001 for her moral courage . The food scandal was heavily discussed in the media.
Brain material from some of the abnormal cattle was then subjected to a histological examination at the Institute for Veterinary Pathology at the University of Veterinary Medicine, Hanover. Spongiform changes were not found. Otherwise the result would have been registered by the veterinary authorities. There was therefore no BSE incident in Bad Segeberg. At that time, German veterinarians were not trained on clinically ill cattle and behavioral disorders in unfamiliar surroundings and after transport stress alone are not sufficient for expressing a suspicion. The complex clinic requires a special neurological examination. As soon as clinical disturbances have occurred, the typical histological changes are regularly determined post-mortem. In this respect, a histological finding would have been made in Hanover with a high degree of probability.
As a result, the legislature reacted with the XEL identifier and gave the official food control through the LFGB new competencies in the field of feed. It is questionable whether the import ban on protein flours from the UK was sufficient. Animal fats obtained at slaughterhouses from the bones and skulls of cattle could also be exported from Great Britain. These fats were processed in milk replacers for calves. Among the clinically normal British slaughter cattle there were certainly some in the incubation phase and the fats were not free of protein. Some of these calves may later have been detected as adults in the official BSE test procedure.
The first cases of BSE in cattle imported from Great Britain occurred in Germany as early as the early 1990s. Four animals from a herd of Galloway and Scottish Highland cattle in Burgdorf near Hanover were examined at the Institute for Veterinary Pathology at the University of Veterinary Medicine Hanover. The remaining stock was then sold to Wagun in Mecklenburg-West Pomerania and a few years later to Brakel in North Rhine-Westphalia. Among them was a Galloway cattle with a forged identity, which was supposed to be born in Wagun, but was an import from Great Britain. This animal became neurologically ill in autumn 1996, died at the end of December and was examined at the state veterinary investigation office in Detmold. The typical histological findings in the brain were recorded at the beginning of January 1997 and the BSE suspicion was confirmed on January 21, 1997 by the Federal Research Center for Virus Diseases in Animals (now the Friedrich Loeffler Institute ). On January 22, 1997, the animal disease officers from the responsible federal and state ministries decided to kill 5,200 cattle imported directly from Great Britain and Switzerland. One of these cattle from Switzerland without a clinic was BSE positive.
Conflicts between conventional cattle farmers and the keepers of robust cattle imported from the UK clearly arose during this period. Every press release about the occurrence of BSE in Germany was associated with uncertainty among consumers. In retrospect, most of the imported Galloway or Highland cattle on the markets in the UK and at the latest during transport were infected with concentrated feed that was not suitable for ruminants. In the UK itself there were hardly any cases of BSE in these cattle due to the natural feeding. In this respect, the local keepers, who wanted to keep them naturally on remaining areas, etc., were innocent of the BSE incident.
It was different in the case of dairy cattle. The cattle feed concentrate available on the common market contained meat-and-bone meal which was increasingly enriched with BSE material as a result of the change in heating in British rendering plants. In addition, economic constraints had led to the increased use of concentrated feed that was not suitable for ruminants.
Experiences with forged identities of cattle in the course of the BSE crisis prompted the federal states to establish the central Hi-Tier database (www.hi-tier.de). The Bavarian State Ministry for Food, Agriculture and Forests (StMELF) was commissioned with this. Among other things, all cattle born in Germany and imported into Germany must be entered here. Your entire curriculum vitae up to death or slaughter is documented in it.
On November 26, 2000, the first BSE case for Germany was announced by the Federal Research Center for Virus Diseases of Animals (today Friedrich-Loeffler-Institut ) (the animal was discovered on November 24, 2000 by a rapid test in Schleswig-Holstein). Up to February 2005, over 360 cases had been officially proven in Germany, 65 of them in 2004 and 54 in the previous year. Since most of the animals were slaughtered before the obligatory examination and before the pathogen could be detected, only 16 cases of BSE were registered in 2006 in around 1.7 million animals examined. In 2007 the registered number of animals tested positive fell to 4, in 2008 only two cattle were tested positive in Germany, both born before 2001. According to the Brandenburg state veterinarian, the last sick animals were reported across Germany in 2009. On January 10, 2014, after five years of rest, a slaughter animal from the Oder-Spree district in Brandenburg was again diagnosed with BSE. The second case of mad cow disease was detected on February 5, 2014 in Lübbecke, East Westphalia, in a cow from the Brandenburg district of Märkisch-Oderland. A connection between the two cases is not known. The Friedrich Loeffler Institute for Animal Health assumed a coincidence.
Austria
The first case occurred in Austria in November 2001, around a year after the BSE rapid tests were introduced across the board. It was an animal imported from the Czech Republic to the Waldviertel ( Lower Austria ). A second case occurred in June 2005 in Vorarlberg's Kleinwalsertal . However, the cattle was born in 1994 . The third case, as it became known on October 28, 2005, occurred in a slaughterhouse in Salzburg . On May 13, 2006, the fourth case became known in a slaughterhouse in the Mühlviertel in Upper Austria . It was a 6 year old cow from a mountain farm. On June 7, 2006, BSE was diagnosed in a 16 year old cow in East Tyrol. Another BSE case became known on November 5, 2006 in Graz . Most recently, on January 19, 2010, another BSE case was found in Upper Austria.
Switzerland
In 1990 the first official case was confirmed in Switzerland . In 1995 the provisional high point was reached with 68 cases and in 1999 another high point with 50 cases. The epidemic was contained through a catalog of measures that was consistently implemented at an early stage.
In 2011 there were again two cases, in 2012 one. Analyzes show that this is not a case of classic BSE, but a so-called atypical BSE. The animal was born in September 2003, two and a half years after the total ban on animal meal feed in Europe.
In January 2020, the first case since 2012 was reported.
United Kingdom
In 1985 and 1986, BSE was first detected in ten cattle in Kent ( England ). But it is possible that BSE had been known for a little longer in Yorkshire under the name stoddy . The number of cases rose to over 36,000 by 1992, and then fell again. Since August 1, 1996, the UK has had a total ban on animal meal feeding for all animal species. Nevertheless, long after 1996, cases of BSE continued to occur in cattle born after the feeding ban. As of July 5, 2006, 134 so-called "BAB" -BSE cows (Born After Ban) have been officially identified.
Up until May 30, 1990, import bans had been imposed on individual British cattle products in 17 countries. France issued a total ban on May 30, 1990.
United States
In the US , the first case was found in late 2003; it was probably an animal imported from Canada about two years earlier . A total of four cases have become known so far. Most recently, in April 2012, it was announced that a dairy cow that had died in California was sick.
Contain the spread
Every case of spongiform encephalopathy, even if it is suspected, must be reported immediately to the responsible veterinary office in Germany . If only one animal in a herd is infected, an attempt is made to prevent the further spread of the infection by culling the entire herd. An unexplained, possibly breed- related increase in Holstein-Friesian cattle should have led to the elimination of the genetic disposition in this breed. However, in most of the cases examined, only the animal that was actually diagnosed with BSE was actually infected. That is why today primarily the offspring of the infected cattle and any herd animals of the same age are killed alone. In Switzerland in particular, this cheaper, so-called cohort sump, has been used since mid-1999.
Since the occurrence of the disease is attributed to infectious concentrate feed, there are now safety regulations for the production of meat and bone meal in affected countries. In England in 1988 it was forbidden to process dead cattle into cattle feed. This measure probably caused the epidemic to decline from 1993.
In addition, the insecticide Phosmet (trade names: Appa, Decemthion, Fosdan, Imidan, Kemolate, Percolate, Prolate, Safidon, Smidan) was suspected of promoting BSE infections or shortening the incubation period. For 1982 to 1992, ie at the time of the accumulated incidence of BSE, many cattle were to fight one in England warble flies high doses -Plage treated with phosmet. The continued use of this agent today in the absence of new infections, however, speaks against this hypothesis.
In the EU, since 2001, the brain, spinal cord and spleen of older animals, organs in which high concentrations of pathogens would be suspected, have to be removed and disposed of at slaughter. This is to minimize the risk of transmission to humans. The processing of beef casings for sausage production is also prohibited in Germany and France, with one exception: Since the disease has not yet been detected in South America, beef casings imported from there can be used in Germany for sausage production.
literature
Scientific
- Report of a WHO Consultation on Public Health Issues related to Human and Animal Transmissible Spongiform Encephalopathies . 1996, WHO / EMC / DIS / 96.147
- Beat Hörnlimann, D. Riesner, H. Kretzschmar: Prions and prion diseases . de Gruyter, Berlin / New York 2001, ISBN 3-11-016361-6 .
- Anonymous: Bovine spongiform encephalopathy (BSE) in cattle and its transferability to humans . Bundesgesundheitsblatt 44 (5), pp. 421-431 (2001), ISSN 1436-9990
- European Communities: Report on the monitoring and testing of ruminants for the presence of transmissible spongiform encephalopathy (TSE) in the EU in 2003, including the results of the survey of prion protein genotypes in sheep breeds . 2004, ISSN 1725-583X , ISBN 92-894-7431-9 . ec.europa.eu (PDF; 6.6 MB)
- B. Hoernlimann, D. Riesner, HA Kretzschmar: Prions in Humans and Animals . de Gruyter, Berlin / New York 2006, ISBN 3-11-018275-0 .
- MJ Prince, JA Bailey, PR Barrowman, KJ Bishop, GR Campbell, JM Wood: Bovine spongiform encephalopathy. In: Revue scientifique et technique de l office international des Epizooties. Volume 22 (1), 2003, pp. 37-60, ISSN 0253-1933
- S. Modrow, D. Falke, U. Truyen: Molecular Virology. 2nd Edition. Spectrum Academic Publishing House / Gustav Fischer Publishing House, Heidelberg / Berlin 2003, ISBN 3-8274-1086-X .
- Ekkehard Schütz, Howard B. Urnovitz, Leonid Iakoubov, Walter Schulz-Schaeffer, Wilhelm Wemheuer, Bertram Brenig: Bov-tA short interspersed nucleotide element sequences in circulating nucleic acids from sera of cattle with bovine spongiform encephalopathy (BSE) and sera of cattle exposed to BSE. In: Clin. Diagn. Lab. Immunol. (2005); 12, pp. 814-820.
Critical
- Irene Soltwedel-Schäfer, Kari Köster-Löscher: The BSE plot: The protocol of calculated insanity . SÖL special edition No. 81, Ecology & Agriculture Foundation, Bad-Dürkheim 2001, ISBN 3-934499-35-X .
- Roland Scholz, Sievert Lorenzen: phantom BSE danger: wrong ways of science and politics in the BSE scandal . Berenkamp Buch- und Kunstverlag, 2005, ISBN 3-85093-193-5 .
- Richard Rhodes: Deadly Meal, a creeping epidemic threatens humanity . SPIEGEL book, Hamburg 1998, original edition New York 1997.
- Variant Creutzfeldt – Jakob Disease in a Patient with Heterozygosity at PRNP Codon 129 . In: N Engl J Med , January 19, 2017, 376, pp. 292-294, doi: 10.1056 / NEJMc1610003
Web links
- Information on BSE and vCJD. WHO status: 2002
- BSE - Deadly Meal . SWR school television, status: July 2002
- BSE: the bugbear's comeback. LifeGen.de, as of September 2006
- Case numbers from the World Organization for Animal Health. ( Memento from January 12, 2011 in the Internet Archive ) As of January 3, 2011
- Information on BSE. German Ministry of Agriculture
- private BSE chronicle as of July 2009
- Monitoring of the national Creutzfeld-Jakob diseases. Website of the University of Edinburgh (English) Status: February 2010 (partly)
- Johann Kirchinger: Bovine Spongiform Encephalopathy (BSE) . In: Historical Lexicon of Bavaria
Individual evidence
- ↑ a b BSE and vCJK (PDF) Robert Koch Institute ; Retrieved February 18, 2012.
- ↑ not used
- ↑ FLI pathogenicity study
- ↑ P. Piccardo, JC Manson, D. King, B. Ghetti, RM Barron: Accumulation of prion protein in the brain that is not associated with transmissible disease. In: Proc. Natl. Acad. Sci. USA . 104 (11), pp. 4712-4717 (2007). PMID 17360589 .
- ↑ RM Barron, SL Campbell, D. King, A. Bellon, KE Chapman, RA Williamson, JC Manson: High titers of transmissible spongiform encephalopathy infectivity associated with extremely low levels of PrP Sc in vivo. In: J Biol Chem . (2007); 282 (49), pp. 35878-35886. PMID 17923484 .
- ↑ N. Makarava, GG Kovacs, R. Savtchenko, I. Alexeeva, H. Budka, RG Rohwer, IV Baskakov: Genesis of mammalian prions: from non-infectious amyloid fibrils to a transmissible prion disease. In: PLoS Pathog . (2011); 7 (12), p. E1002419. PMID 22144901 .
- ↑ C. Bett, S. Joshi-Barr, M. Lucero, M. Trejo, P. Liberski, JW Kelly, E. Masliah, CJ Sigurdson: Biochemical properties of highly neuroinvasive prion strains. In: PLoS Pathog. (2012); 8 (2), p. E1002522. PMID 22319450 .
- ↑ RN Rambaran, LC Serpell: Amyloid fibrils: abnormal protein assembly. In: Prion. (2008); 2 (3), pp. 112-117. PMID 19158505 .
- ↑ NJ Cobb, WK Surewicz: prion diseases and Their biochemical mechanisms. In: Biochemistry . (2009); 48 (12), pp. 2574-2585. PMID 19239250 .
- ↑ a b M. Imran, S. Mahmood: An overview of animal prion diseases. In: Virol J. (2011), Volume 8, p. 493. PMID 22044871 ; PMC 3228711 (free full text).
- ↑ John Haybaeck u. a .: Aerosols Transmit Prions to Immunocompetent and Immunodeficient Mice. In: PLoS Pathogens. (2011); 7 (1), p. E1001257, doi: 10.1371 / journal.ppat.1001257
- ↑ J. Bertram, M. Mielke, M. Beekes, K. Lemmer, M. Baier, G. Pauli: Inactivation and removal of prions in the preparation of medical products. A contribution to the examination and declaration of suitable procedures. In: Federal Health Gazette - Health Research - Health Protection. (2004) Volume 47, p. 1, pp. 36-40. Retrieved February 18, 2012.
- ↑ A. Sakudo, Y. Ano, T. Onodera, K. Nitta, H. Shintani, K. Ikuta, Y. Tanaka: Fundamentals of prions and their inactivation. (review). In: Int J Mol Med. (2011), Volume 27 (4), pp. 483-489. doi: 10.3892 / ijmm.2011.605 . PMID 21271212 .
- ^ Continuing education course EU reference laboratory at the VLA in Weybridge, 1997
- ↑ MA Barria, D. Gonzalez-Romero, C. Soto: Cyclic amplification of prion protein misfolding. In: Methods Mol Biol. (2012), Volume 849, pp. 199-212. PMID 22528092 .
- ↑ PM Gordon, E. Schütz, J. Beck, HB Urnovitz, C. Graham, R. Clark, S. Dudas, S. Czub, M. Sensen, B. Brenig, MH Groschup, RB Church, CW Sensen: Disease- specific motifs can be identified in circulating nucleic acids from live elk and cattle infected with transmissible spongiform encephalopathies. In: Nucleic Acids Res . (2009), Volume 37 (2), pp. 550-556. PMID 19059996 ; PMC 2632913 (free full text).
- ↑ USA: First BSE case in six years. In: aerztezeitung.de, April 25, 2012; accessed on March 5, 2014.
- ↑ BSE numbers hit new low. In: Dt. TAB. Issue 11 (2008), p. 1543.
- ↑ BMEL: Number of confirmed BSE cases in Germany , on bmel.de, accessed on September 27, 2018.
- ↑ Annett Heide: What is Margrit Herbst doing? In: Stern , No. 48/2019, p. 122.
- ^ Dieter Deiseroth : Whistleblowing in times of BSE. The case of the veterinarian Dr. Margrit Herbst. Berlin Verlag, Berlin 2001, ISBN 3-8305-0258-3
- ^ Continuing education course EU reference laboratory at the VLA in Weybridge, 1997
- ↑ heynkes.de
- ↑ Cattle epidemic: The chronology of the BSE crisis . Spiegel Online , November 28, 2000; accessed on March 5, 2014
- ↑ BSE is defeated - almost . Zeit Online , April 21, 2009; accessed on March 5, 2014.
- ↑ BSE discovered in cattle in Brandenburg . n24.de, January 2, 2014; accessed on March 5, 2014.
- ↑ Mad cow disease discovered in NRW . In: Berliner Zeitung , February 2, 2014.
- ↑ BSE back in Austria after two years: cow in Upper Austria with positive test
- ↑ Federal Food Safety and Veterinary Office
- ↑ Statistics Bovine Spongiform Encephalopathy (BSE)
- ↑ Dangerous animal disease - Swiss cow infected with BSE - authorities give the all-clear. In: srf.ch . February 6, 2020, accessed February 7, 2020 .
- ↑ USA: First BSE case in six years. on: aerztezeitung.de , April 25, 2012.
- ↑ Ordinance on the meat hygiene examination of slaughtered cattle for BSE (BSEUntersV)

