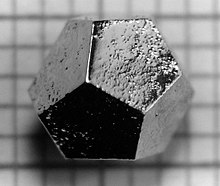
Quasicrystal
In quasicrystals , the atoms or molecules are arranged in an ordered but aperiodic structure . They were discovered experimentally in 1982 by Daniel Shechtman , who was awarded the Nobel Prize in Chemistry in 2011 . The name Shechtmanite goes back to his discovery . Mathematically, these structures were first described by Peter Kramer and Roberto Neri in 1984. Paul Steinhardt and Dov Levine in 1984 also contributed significantly to their structure elucidation .
history
In 1982 Dan Shechtman discovered an unusual structure in the diffraction pattern ( X-ray or electron diffraction ) during the crystal structure analysis of a rapidly cooled aluminum - manganese alloy (14% manganese ). This possessed the point group symmetry m 3 5 and thus the symmetry of an icosahedron . This is very unusual for crystalline substances, since with this symmetry no lattice shifts are possible and therefore no periodic structure, as is necessary for the definition of a crystal , is present. In mathematical terms , icosahedral quasicrystals were first constructed in 1984 by Peter Kramer and Roberto Neri. Later Dov Levine and Paul Joseph Steinhardt coined the term “quasicrystal” for this new phase type and compared the data found during structural analysis with mathematical models.
An early pioneer of quasicrystals was Alan Mackay in Great Britain, who published a paper on icosahedral spherical packing (a packing with five-fold symmetry) and Penrose tiling in crystallography in the early 1980s in the early 1980s. For this he received the Oliver E. Buckley Condensed Matter Prize with Levine and Steinhardt in 2010 .
Pattern in quasicrystals
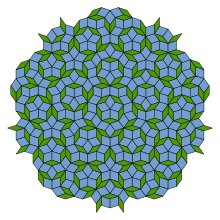
In a normal crystal the atoms or molecules are arranged in a periodic structure. This structure is repeated in each of the three spatial directions, similar to how honeycombs are repeated in two spatial directions. Each cell is surrounded by cells that form an identical pattern. In a quasicrystal, however, the atoms or molecules are only arranged “quasiperiodically”. The atoms are locally in a regular structure, but on a global scale the structure is aperiodic, each cell is surrounded by a different pattern.
What is particularly remarkable about the quasicrystals is that they have a five-, eight-, ten- or twelve-fold symmetry . In a normal crystal, only one, two, three, four and sixfold symmetries are possible. This results from the fact that the space can only be filled with congruent parts in this way . Before quasicrystals were discovered, it was assumed that fivefold symmetry could never occur because it was not possible to periodically fill space.
The discovery of quasicrystals helped redefine what the essence of a crystal is. Quasicrystals do not have periodic structures, but they have sharp diffraction points. There is an important relationship between quasicrystals and the Penrose tiling that Roger Penrose had already found before the quasicrystals were discovered: if you cut a quasicrystal properly, the cut surface will show exactly the pattern of the Penrose tiling.
Geometric interpretation
A periodic pattern can be shifted completely by a certain distance so that each shifted atom takes exactly the place of a corresponding atom in the original pattern . In a quasi-periodic pattern, such a complete parallel shift of the pattern is not possible, regardless of the distance chosen. However, you can move any section, regardless of its size, so that it (possibly after a rotation) is congruent with a corresponding section.
There is a relationship between periodic and non-periodic patterns. Any quasi-periodic pattern of points can be formed from a periodic pattern of a higher dimension: for example, to create a three-dimensional quasi-crystal, one can start with a periodic arrangement of points in a six-dimensional space . The three-dimensional space is a linear subspace that penetrates the six-dimensional space at a certain angle. If you project every point of the six-dimensional space that is within a certain distance from the three-dimensional sub-space onto the sub-space and the angle represents an irrational number, such as the golden section , then a quasi-periodic pattern is created.
Any quasi-periodic pattern can be created in this way. Any pattern obtained in this way is either periodic or quasi-periodic. This geometrical approach is useful for analyzing physical quasicrystals. In a crystal, lattice defects are places where the periodic pattern is disturbed. In a quasicrystal these are places where the three-dimensional subspace is bent, folded or broken when it penetrates the higher-dimensional space.
Occurrence
Quasicrystals occur in many three-dimensional alloy systems. Most alloys that contain quasicrystals are thermodynamically unstable, so they can only be formed by rapid cooling and, when heated again, transform into more stable crystals. However, there are also a number of thermodynamically stable alloys with a quasi-crystalline structure. These are usually ternary alloys, i.e. those with three alloy elements and the elements aluminum , zinc , cadmium or titanium as the main component. These alloys - and those with “neighboring” concentrations - are often amorphous (or initially amorphous, before the actual crystallization). Amorphous systems are therefore often competitors to quasicrystals (competition between so-called α-phases and so-called i- phases).
The rare two-element systems with a quasi-crystalline structure include Cd 5.7 Yb, Cd 5.7 Ca in an icosahedral structure and Ta 1.6 Te in a dodecahedral structure. Since the quasicrystalline structure is usually only stable within a very narrow range of the elements in which the elements are mixed, quasicrystals can also be counted among the intermetallic compounds .
So far only one naturally occurring quasi-crystalline mineral , the icosahedrite , is known. It is an aluminum-copper-iron alloy with the composition Al 63 Cu 24 Fe 13 , which was found on the Chukchi Peninsula in Russia .
use
Various applications of quasi-crystalline compounds are being investigated. This applies to both composite materials, in which quasi-crystalline compounds are added to alloys to improve certain properties, and quasi-crystalline coatings. As an additive to steel , quasicrystals enable a very strong, ductile, corrosion and aging-resistant steel. It is particularly interesting for medical devices, for example in surgery or acupuncture. The properties of aluminum alloys, such as strength at high temperatures or deformability, can also be improved by adding quasicrystals.
Quasi-crystalline coatings enable particularly low abrasion and low adhesion due to their hardness and oxidation stability. This is of interest for frying pans , so quasi-crystal coatings are being investigated as an alternative to stainless steel pans and polytetrafluoroethylene coatings.
In catalysis, too, quasi-crystalline compounds are being investigated as possible catalysts , such as a quasi-crystalline aluminum-copper-iron alloy for the steam reforming of methanol .
literature
- A. Katz: A Short Introduction to Quasicrystallography . In: Waldschmidt (editor): From Number theory to physics . Springer, 1992, doi : 10.1007 / 978-3-662-02838-4_11 .
- Michael Baake, Robert Moody , Uwe Grimm: Hidden Order of Quasicrystals . In: Spectrum of Sciences . February 2002, pp. 64-74. English: What is Aperiodic Order? In: arXiv. 2002, arxiv : math.HO / 0203252 .
- C. Giacovazzo (Ed.): Fundamentals of Crystallography. Oxford University Press, 1992, ISBN 0-19-855578-4 , pp. 223-226.
- Charles Radin : Quasicrystals and Geometry . In: Notices AMS. 43, 1996, 416.
- Charles Radin: A revolutionary material . In: Notices AMS. 60, No. 3, 2013, 310.
- Walter Steurer , Sofia Deloudi: Crystallography of Quasicrystals: Concepts, Methods and Structures, Springer 2009
Web links
- Matthias Hullin: Quasicrystals: Discovery, Materials, Properties
- Forbidden crystals / A mathematical idea stood at the beginning of a new class of materials. In: Marburger UniJournal 6/2000
- Alexandra Ledermann, Georg von Freymann, Martin Wegener: Laue diffraction on the desk. Photonic quasicrystals. In: Physics in Our Time. 38, 2007, p. 300, doi : 10.1002 / piuz.200601153 .
- Alexandra Ledermann: Design, Fabrication, and Characterization of Three-Dimensional Photonic Quasicrystals. Karlsruhe Institute of Technology , 2010
- 2011 documentary about research at the University of Stuttgart on quasicrystals
- Charles Radin: Quasicrystals, Scholarpedia
Individual evidence
- ↑ B Ünal, V. Fournée, KJ Schnitzenbaumer, C. Ghosh, CJ Jenks, AR Ross, TA LoGrasso, JW Evans, PA Thiel: nucleation and growth of Ag islands on fivefold Al-Pd-Mn quasi crystal surfaces: Dependence of Iceland density on temperature and flux . In: Physical Review B . 75, 2007, p. 064205. doi : 10.1103 / PhysRevB.75.064205 .
- ^ The Nobel Prize in Chemistry 2011. Nobelprize.org (official homepage of the Nobel Prize), accessed on October 5, 2011 (English).
- ^ D. Shechtman, I. Blech, D. Gratias, J. Cahn: Metallic Phase with Long-Range Orientational Order and No Translational Symmetry. In: Physical Review Letters. 53, 1984, pp. 1951-1953, doi: 10.1103 / PhysRevLett . 53.1951 .
- ^ P. Kramer, R. Neri: On periodic and non-periodic space fillings of E (m) obtained by projection . In: Acta Crystallographica . A40, No. 5, 1984, p. 580. doi : 10.1107 / S0108767384001203 .
- ^ Dov Levine, Paul Steinhardt: Quasicrystals: A New Class of Ordered Structures. In: Physical Review Letters. 53, 1984, pp. 2477-2480, doi: 10.1103 / PhysRevLett.53.2477 .
- ↑ Entry on quasicrystals. In: Römpp Online . Georg Thieme Verlag, accessed on October 5, 2011.
- ↑ a b c d Enrique Maciá, Jean-Marie Dubois, Patricia Ann Thiel: Quasicrystals . In: Ullmann's Encyclopedia of Technical Chemistry . Wiley-VCH, Weinheim 2008, doi : 10.1002 / 14356007.e22_e01.pub2 .
- ↑ Luca Bindi, Paul J. Steinhardt, Nan Yao, Peter J. Lu: Natural Quasicrystals. In: Science 324, No. 5932, 2009, pp. 1306-1309, doi: 10.1126 / science.1170827 .
- ↑ Quasicrystals. Reaching maturity for technological applications (page 5, lib.dr.iastate.edu, accessed June 3, 2016)