Reproterol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
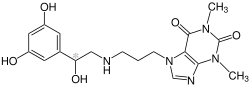
|
||||||||||||||||||||||
| Mixture of stereoisomers - structural formula without stereochemistry | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Reproterol | |||||||||||||||||||||
| other names |
( RS ) -7- (3 - {[2- (3,5-dihydroxyphenyl) -2-hydroxyethyl] amino} propyl) -1,3-dimethyl-3,7-dihydro-1 H -purine-2,6 -dion ( IUPAC ) |
|||||||||||||||||||||
| Molecular formula |
|
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | ||||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
249–250 ° C (reproterol monohydrochloride) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Reproterol is a drug from the group of β 2 sympathomimetics and is used as a racemate . Administered as a metered aerosol or injection solution, it dilates the bronchi and is used for the treatment of bronchial asthma.
pharmacology
application areas
Reproterol is used as a metered dose aerosol in combination with cromoglicinic acid to prevent and treat dyspnoea in asthma in patients who require bronchodilator therapy in addition to basic anti-inflammatory therapy. Reproterol solution for injection is given intravenously for the short-term treatment of a severe asthma attack.
Mechanism of action
Reproterol, which has a half-life of 1.5 hours, acts as an agonist on the sympathetic β 2 -adrenoceptors to relaxation (slackening) of the smooth muscles of the bronchi and thus to a reduction in breathing resistance.
Side effects
As has been observed with β 2 -sympathomimetics, side effects on the cardiovascular system ( tachycardia , palpitations) can occasionally occur. In addition, tremors, restlessness and a rise in blood sugar can be observed.
Interactions
Other β-sympathomimetics, theophylline and anticholinergics increase the cardiovascular side effects of reproterol. MAOIs slow down the breakdown of reproterol.
Stereoisomerism
Reproterol is chiral , so it contains a stereocenter. There are thus two enantiomers , the ( R ) form and the ( S ) form. The commercial preparations contain the drug as a racemate (1: 1 mixture of enantiomers).
| Enantiomers of reproterol | |
|---|---|
 CAS number: 210710-33-1 |
 CAS number: 210710-34-2 |
Manufacturing
A synthesis for reproterol, starting from theophylline , is described in the literature.
Other Information
Due to its mechanism of action, Reproterol is on the prohibited list of the World Anti Doping Code and is therefore a prohibited doping agent in sport. The use of drugs containing reproterol must be reported to the doping control.
Trade names
Bronchospasm (D)
Aarane (D), Allergospasmin (D)
Individual evidence
- ^ The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals . 14th edition, 2006, p. 1402, ISBN 978-0-911910-00-1 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b Entry on Reproterol. In: Römpp Online . Georg Thieme Verlag, accessed on May 29, 2014.
- ↑ Rote Liste Service GmbH (Ed.): Rote Liste 2017 - drug directory for Germany (including EU approvals and certain medical devices) . Rote Liste Service GmbH, Frankfurt / Main, 2017, edition 57, ISBN 978-3-946057-10-9 , p. 196.
- ^ Axel Kleemann , Jürgen Engel, Bernd Kutscher and Dietmar Reichert: Pharmaceutical Substances, 4th edition (2000), 2 volumes published by Thieme-Verlag Stuttgart, ISBN 978-1-58890-031-9 ; online since 2003 with biannual additions and updates.
- ↑ Red List Online, as of August 2009