Ropinirole
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
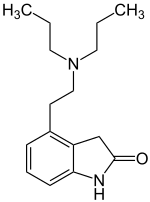
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Ropinirole | |||||||||||||||||||||
| other names |
4- (2-Dipropylaminoethyl) -1,3-dihydroindol-2-one ( IUPAC ) |
|||||||||||||||||||||
| Molecular formula | C 16 H 24 N 2 O | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 260.37 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
241–243 ° C (ropinirole hydrochloride ) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Ropinirole is a drug from the group of dopamine agonists . It is primarily used in the treatment of Parkinson's disease and restless legs syndrome . It is structurally similar to dopamine and (unlike many other dopamine agonists) does not belong to the ergot alkaloids .
It is not as effective as levodopa as monotherapy , but it is more effective than bromocriptine . In combination therapy it can replace part of the L-Dopa administration. The adverse drug effects typical of all dopamine agonists include: a. Nausea, circulatory disorders and water retention in the legs. In contrast to L-Dopa, ropinirole is said to have a significantly lower tendency to develop movement disorders (dyskinesia) in long-term therapy.
pharmacology
Mode of action
Ropinirole is a dopamine agonist. Although it has a different chemical structure than dopamine, because it attaches to the same binding positions, it has a comparable effect. Since it can cross the barrier to the brain unlike externally supplied dopamine without difficulty, ropinirole in tablet form is a good way to compensate for the dopamine deficiency in patients with Parkinson's and Restless Legs Syndrome.
Difference from other dopamine agonists
The effectiveness of ropinirole is comparable with the combination therapy bromcriptine with selegiline .
Contraindications
Ropinirole must not be used in severe kidney and liver disorders or in diseases of the cardiovascular system . Dosing should be cautious in patients with a history of psychiatric treatment .
Interactions
The effect of ropinirole is enhanced by quinolone antibiotics and high-dose estrogen preparations.
Side effects
Due to the occurrence of possible "sleep attacks", driving vehicles or carrying out work with a potential risk of injury while using nonergoline dopamine agonists (pramipexole, ropinirole) is prohibited.
The term “sleep attack” denotes an increased imperative need for sleep with, however, also reduced vigilance and increased sleepiness and is not identical with the term actual sleep attack in narcolepsy. It should also be taken into account that sleep disorders occur as a result of Parkinson's disease and that "sleep attacks" can also occur in healthy people. However, since treatment with pramipexole and ropinirole leads to increased indications of the above If you have had sleep attacks, driving a car is not recommended. A pathophysiological connection with the activation of D3 receptors is discussed.
Nausea and vomiting , abdominal pain, reflux oesophagitis , dizziness , drop in blood pressure when standing up (orthostatic hypotension ), daytime sleepiness , movement disorders (dyskinesia), water retention in the legs ( edema ) and hallucinations (symptomatic pharmacotoxic psychosis ) are the main side effects of ropinirole.
As with all dopamine agonists , stimulating the reward system can lead to obsessive-compulsive disorder or reduced impulse control in certain patients , which can lead, for example, to addiction to gambling , shopping frenzy , binge eating , internet addiction and hypersexuality , the latter also changing sexual behavior into highly taboo acts up to exhibitionism , supposedly Can change pedophilia or homosexuality . The effects may depend on the dose and symptoms disappear after discontinuation. This effect has been known for some time, but it was pointed out in 2004 that it was not mentioned in any product information for a dopamine agonist. In the case of Requip , this information has only been included on the package insert since 2006, after a patient complained in France who took the drug from 2003 to 2005. In another study, however, ropinirole was used (successfully) as an antidote for loss of libido under treatment with antidepressants.
Since there is no ergot having structure, other than any pleuropulmonary and retroperitoneal in Mutterkornalkaloidpräparaten fibrosis to fear or peripheral vascular side effects.
The treatment of restless legs syndrome with ropinirole can worsen the symptoms (so-called rebound or augmentation effects ). Similar interfering effects are known from L-dopa and other dopamine agonists.
Warning notices
During long-term therapy with ropinirole, the attending physician must carry out check-ups at regular intervals. Sudden discontinuation can lead to a severe deterioration in health. In pregnancy and during breastfeeding Ropinirole should not be used.
Potency and dosage
Ropinirole is available in tablet form in strengths of 0.25, 0.5, 1, 2, 4, 5 and 8 mg.
At the beginning of the treatment, the dosage is slowly increased from 0.25 mg over several weeks to a standard maintenance dose of 3 to 9 mg per day and can be increased to max. 24 mg per day can be increased.
Manufacturing
A multi-step synthesis for ropinirole, starting from benzoyl chloride and isochroman , is described in the literature.
Trade names
Adartrel (D, CH), Requip (D, A, CH), Rolipexa (A), Ropinal (D), numerous generics (D, A)
Individual evidence
- ^ The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals. 14th edition, 2006, pp. 1427-1428, ISBN 978-0-911910-00-1 .
- ↑ a b Data sheet Ropinirole hydrochloride from Sigma-Aldrich , accessed on April 22, 2011 ( PDF ).
- ^ JC Möller, K. Stiasny, W. Cassel, JH Peter, HP Krüger, WH Oertel: “Sleep attacks” in Parkinson's patients A side effect of nonergoline dopamine agonists or a class effect of dopamimetics? In: The neurologist . tape 71 , no. 8 , August 2000, p. 670-676 , doi : 10.1007 / s001150050645 .
- ↑ Addiction disorders caused by dopamine agonists , pharmische-zeitung.de , August 31, 2010.
- ↑ Hypersexuality and gambling addiction among dopamine agonists ( memento of April 13, 2009 in the Internet Archive ), aerzteblatt.de, April 9, 2009.
- ^ Dopamine agonist gambling addiction , aerzteblatt.de, February 13, 2007.
- ↑ Hypersexuality under the dopamine agonist pramipexole ( Memento of March 18, 2011 in the Internet Archive ), arznei-telegramm 3/2004; 35:36.
- ↑ Father claims that drugs triggered his homosexuality , lesbian.or.at, December 11, 2007.
- ↑ Man claims Glaxo drug made him gay sex addict, says report . CBS News January 31, 2011
- ^ JJ Worthington III, NM Simon, NB Korbly, RH Perlis, MH Pollack: Ropinirole for antidepressant-induced sexual dysfunction . In: International Clinical Psychopharmacology . tape 17 , no. 6 , November 2002, pp. 307-310 , PMID 12409684 .
- ↑ Restless Legs (RLS): Dopamine antagonists are now also approved . In: arznei-telegram . tape 37 , no. 7 , 2006, p. 62–63 ( arznei-telegramm.de ).
- ↑ ABDA database (as of July 29, 2008).
- ^ Axel Kleemann , Jürgen Engel, Bernd Kutscher and Dietmar Reichert: Pharmaceutical Substances, 4th edition (2000), 2 volumes published by Thieme-Verlag Stuttgart, ISBN 978-1-58890-031-9 ; online since 2003 with biannual additions and updates.