Hydrogen atom

A hydrogen atom is an atom of the chemical element hydrogen (symbol: H). The atom consists of a single positively charged nucleus (with one proton and, in the case of naturally occurring isotopes, zero to two neutrons ) and a negatively charged electron . Electron and atomic nucleus are bound to one another due to their opposite electrical charge ( Coulomb's law ).
The hydrogen atom is the most simply structured of all atoms and provides the key to understanding the structure and properties of all atoms . It is the only atom for which the quantum mechanical Schrödinger equation is analytical, i.e. H. can be solved in a mathematically closed form. The spectral lines of the hydrogen atom can be calculated with high accuracy and can be compared with the measured values, such as B. the most famous lines multiplet , the Balmer series .
The hydrogen atom as a prime example of the development of atomic physics
The principles of atomic physics , which are gradually derived from the hydrogen atom, form the basis for describing all atoms today.
Before 1913
William Prout hypothesized in 1815 that the atoms of all matter are composed of hydrogen atoms. As a justification, he stated that, according to John Dalton , hydrogen has the smallest of all atomic weights (also correct today) and the atomic weights of other elements are an integral multiple of this - this was, however, already wrong according to what was known at the time, for the reason see Isotopes . According to today's view, this building process actually takes place during nucleosynthesis in supernovae.
In 1885, Johann Balmer found a mathematical relationship between the wavelengths of the four visible spectral lines of the hydrogen atom that could be represented using simple whole numbers, the Balmer formula . After the discovery (by Robert Wilhelm Bunsen and Gustav Robert Kirchhoff in 1860) that every element shows specific spectral lines, he assumed that an explanation for this would most likely be found in the case of hydrogen, because it has the lightest atomic weight and the simplest Line spectrum shows. Balmer used his formula to predict the exact location of further lines in the ultraviolet, and then learned, to his surprise, that these had already been found precisely there in star spectra.
Joseph John Thomson identified the electron as a universal component of all matter in 1897 and in 1903 formulated a model of the atom with a massless positive charge the size of the atom and a sufficient number of electrons to explain the atomic mass, for the H atom no less than about 2000 The electrons should be distributed in the positive charge like “ the raisins in the cake ” and be able to swing around their position of rest, which made the emission and absorption of light of certain frequencies explainable in the context of classical physics, as well as the scattering of light and X-rays . Particular attention was paid to the fact that this model could also explain the splitting of the light frequency in the magnetic field, at least in those cases in which one saw a triple splitting (normal Zeeman effect , 1896). It remained inexplicable how such an arrangement could be as stable as the atoms are obviously. In addition, (from 1906) the scattering of X-rays turned out to be much weaker than predicted, which speaks for a much smaller number of electrons in the atom: for hydrogen, at most a few.
Arthur Haas showed for the first time in 1910 that, with the aid of Planck's constant h, discovered in 1900, a formula can be set up that gives correct values for the radius and the binding energy of the hydrogen atom. This was the first application of Planck's formula, which until then had been used almost exclusively for light waves and other harmonic oscillations, to a problem in the mechanics of the atom - and it was largely rejected for precisely this reason. Haas looked at an electron (charge -e ) on a circular path on the surface of a positively charged sphere with the charge + e and put its potential energy E pot and rotational frequency f , both determined according to classical physics, into the ratio . The result corresponds exactly to the basic state of Bohr's model.
1913-1925
In 1913, Niels Bohr showed in his Bohr atomic model that with the assumption of a small, positive, heavy atomic nucleus (based on Ernest Rutherford's discovery in 1911) and further assumptions (later refined and called Bohr's postulates ), the energies of the H atom also calculated correctly in excited states can be. Although these postulates meant radical breaks with classical physics, the model quickly caught on because it could not only use the Balmer formula, but also the spectrum of the ionized helium atom (an electron orbits an atomic nucleus with twice its charge and four times its mass) Explain correctly, including the small effect of the various nuclear masses, as well as the energies of the hard X-rays of the heavier elements.
In 1916 Arnold Sommerfeld expanded the model to include elliptical orbits to create Bohr-Sommerfeld's atomic model . He discovered the three spatial quantum numbers and the directional quantization of the angular momentum, so that he could explain the (odd-numbered) splitting of the energy levels in the magnetic field . By considering the special theory of relativity, further subtleties of the spectral lines of the H atom could be interpreted. However, like Bohr's model, the model failed with atoms with several electrons.
From 1925
Quantum mechanics
The breakthrough to today's model came in 1925/26 with the discovery of quantum mechanics by Werner Heisenberg ( matrix mechanics ) and Erwin Schrödinger ( wave mechanics ). The new form of equations of motion make Bohr's postulates superfluous. The touchstone of the new theories was the exact calculation of the H atom. Wolfgang Pauli succeeded for the first time with the matrix mechanics . The first calculation according to wave mechanics (see below), which provides the geometric shape of the electron orbitals in addition to the energies , was presented by Schrödinger himself. Systems with more than one electron can no longer be treated with mathematical precision here either, but approximation methods make it possible to calculate the behavior of atoms with many electrons with high accuracy. The quantum mechanical model led to the shell model and thus to an understanding of the structure of the periodic table of the elements and the formation of molecules in the chemical bond .
Relativistic quantum mechanics
When Paul Dirac published the relativistically correct formulation of quantum mechanics in 1927 , he was not only able to explain further subtleties of the spectral lines of the hydrogen atom, but also the previously incomprehensible properties of the electron such as spin and anomalous magnetic moment . This also understood the even-numbered splitting of the energy levels in the anomalous Zeeman effect . In addition, the Dirac equation resulted in the new prediction of antiparticles .
Quantum electrodynamics
The vacuum polarization theoretically expected in the context of quantum electrodynamics by virtual particle-antiparticle pairs was first demonstrated on H atoms in 1947 using an extremely small splitting of an energy level ( Lamb shift ). This helped quantum electrodynamics, in particular the renormalization process required therein , to achieve a breakthrough. That quantum electrodynamics also offers an explanation for classical electrostatic attraction through the exchange of virtual photons was shown in 1952 by Hans Bethe and E. Salpeter on the basis of another precise calculation of the energy levels of the H atom ( Bethe-Salpeter equation ).
Dynamic group
During his first treatment of the hydrogen atom in Heisenberg's matrix mechanics, Wolfgang Pauli had found a way to take into account the exact properties of the Coulomb potential simply by adding an additional constant, the Laplace-Runge-Lenz vector . Mathematically, this corresponds to a rotational symmetry in 4 dimensions ( symmetry group O (4)). By expanding to the dynamic symmetry group O (4,2), Hagen Kleinert was able to combine not only the energy terms, but also the dipole matrix elements for all atomic transitions in an irreducible group representation.
Path integral
In 1979, Hagen Kleinert found a solution for the hydrogen atom in Feynman's path integral approach to quantum mechanics and thus expanded the scope of this method, which is important in modern quantum mechanics.
Experimental investigations of the optical line spectra
The spectral lines in the absorption and emission spectrum of the hydrogen atom have been investigated since 1862 . They are caused by the transition of the bound electron from a higher to a lower level (emission) or vice versa (absorption). The possible lines are classified according to the basic level and named after their respective discoverers. Mathematically, the transitions have been described by the Rydberg formula since 1888 . Albert A. Michelson found in 1892 that the spectral lines show fine splits. Some of these could only be explained in 1916 by Bohr-Sommerfeld's atomic model , and completely from 1926 by quantum mechanics .
| Name of the series | Basic level | Wavelength range | Discovery year |
|---|---|---|---|
| Lyman series | 1 | ultraviolet | 1906 |
| Balmer series | 2 | visible to ultraviolet | 1885 |
| Paschen series | 3 | infrared | 1908 |
| Brackett series | 4th | infrared | 1922 |
| Pound series | 5 | infrared | 1924 |
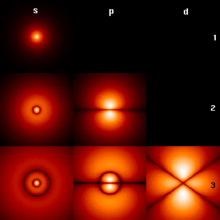
Solution of the Schrödinger equation (hydrogen problem)
The simplest Schrödinger equation for a charged particle in the Coulomb field of a point charge is called the hydrogen problem. This three-dimensional partial differential equation can be separated into three independent equations due to the spherical symmetry of the electromagnetic interaction . Each of the three individual equations can be solved mathematically exactly.
One of the equations gives the form of the distance dependence of the electron wave function in the energy states and the energy values of the electron in the hydrogen atom. It is common to refer to the various discrete energy values over the principal quantum number as . The deepest state of energy is called .
The other two equations contain the angular dependence of the wave function with the orbital angular momentum quantum number and the magnetic quantum number .
The hydrogen atom is one of the few quantum mechanical systems that can be precisely calculated. The solution of the Schrödinger equation for the hydrogen atom is therefore a standard example of university physics and chemistry education.
Mathematical details
For the system of an electron (mass , location , charge ) and the atomic nucleus (mass , location , charge , where is an integer), where the Coulomb interaction determines the potential energy, the time-independent Schrödinger equation applies :
- .
The Laplace operators are the second spatial (partial) derivatives according to the coordinates or . With the transition to the center of gravity system and consideration of the relative movement ( ) of the electron and atomic nucleus, the following Schrödinger equation results after separation:
- .
It is the reduced mass of the system. This can be due by approach. This equation is otherwise identical to the Schrödinger equation of a particle in a spherically symmetric potential. The separation of this equation into spherical coordinates (distance from the center point), (latitude angle) and (longitude angle) leads to three equations, each of which depends on only one of the coordinates. A complete solution is given by the product of the solutions to these three equations.
Every solution of the Schrödinger equation for the hydrogen atom is identified by the three whole numbers . They are called quantum numbers, more precisely: spatial quantum numbers in contrast to the later added spin quantum number . The main or energy quantum number is any positive number, the angular momentum quantum number takes on the values from to for a given , and the magnetic quantum number runs through the whole numbers from to for a given . The solution function is then
With
- (therein is the Bohr radius of the H atom);
- are the associated Laguerre polynomials ;
- are the spherical surface functions .
The radial function is the same for all values of the magnetic quantum number . The angle-dependent functions are independent of the principal quantum number.
For the lowest orbitals we get:
The energy eigenvalues are
With
with the Rydberg energy .
The eigenvalues for angular momentum quantum number and magnetic quantum number are through
and
given.
The angular momentum quantum number in this case measures the path of angular momentum of the electron, and the magnetic quantum number its projection on an arbitrarily defined direction, which is generally as such is referred to (z stands for the z-axis).
In this simplest treatment of the hydrogen atom, the energy values only depend on the principal quantum number. All states with the same (and thus also all their possible linear combinations) have the same energy here. It is therefore said that they are degenerate with respect to the quantum numbers and
The degeneracy with regard to generally applies to every spherically symmetric potential, because then the energy of an eigenstate cannot depend on the orientation of the angular momentum with regard to the -axis. On the other hand, the degeneracy is a special feature of the ( ) -potential. It explains the particularly high electrical polarizability (linear Stark effect ) of the excited hydrogen atom. In atoms with several electrons, the degeneracy has already broken up due to the change in the potential form due to the distance-dependent shielding of the nuclear charge by the other electrons, which is of great importance for the structure of the periodic table of the elements. However, the degeneracy always applies (to a good approximation) when an electron is at a large distance from all the others, i.e. in a Rydberg state . Further effects (spin, relativity theory) result in a slight cancellation of the degeneracy even in the H atom.
- ↑ The lowest energy state (ground state) is usually referred to in quantum mechanics .
Further development
The Schrödinger equation gives a first approximation of an excellent description of the hydrogen atom. However, it neglects some facts that can be proven experimentally and can be derived strictly formally within the framework of a relativistic treatment . Some of these corrections can be explained by modifying the non-relativistic equation:
Relativistic Effects
The Schrödinger equation is a non-relativistic equation and uses the classical expression for kinetic energy. For a correct description one must therefore use a relativistic equation for the development over time. In the case of the hydrogen atom, however, the difference in energy is not very large, so the effects can be treated in terms of perturbation theory within the framework of the Schrödinger equation in order to obtain more precise values for the energy eigenvalues.
Spin
The spin of the electron is also a non-classical effect in the relativistic Dirac equation can be understood that for fermions with spin value is tailored.
Because of its spin, the electron has a magnetic moment that can interact with other magnetic moments and magnetic fields.
The interaction with the magnetic moment, which the electron generates through its movement around the atomic nucleus, is called spin-orbit coupling . This and other effects cause a partial splitting of the degenerate energy levels, the fine structure of the hydrogen atom. If one also takes into account the interaction of spin and orbital angular momentum with the nuclear spin , an even finer split results, the so-called hyperfine structure .
In the presence of magnetic fields, the anomalous Zeeman effect is observed due to the spin of the electron .
Quantum field theoretical effects
Even the description of an electron using the relativistic Dirac equation with so-called minimal coupling to an electromagnetic field leaves many physical and mathematical problems unsolved.
Only relativistic quantum field theories were able to explain all known and measurable properties of the hydrogen atom (and that of all other atoms) and in principle make them calculable from natural constants and elementary particle properties . The QFT are many-particle theories, in which, by means of a second quantization, all forces are explained by interaction particles, whereby new effects such as vacuum fluctuations are described. These involve, for example, the Lamb shift (Engl. Lamb shift ) of the energy levels, which for the first time in the Lamb-Retherford experiment has been demonstrated, as well as corrections to the gyromagnetic factor . The spontaneous emission of photons from excited states of the atom - including the transition probabilities - could only be properly explained with the help of quantum electrodynamics.
The quantum electrodynamics , a quantum field theory in which the effect of the electromagnetic field ultimately photons "light particles", is recycled, provides currently the most accurate model of the electron shell of a hydrogen atom.
literature
- Abraham Pais: Inward Bound: Of Matter and Forces in the Physical World . Clarendon Press, Oxford 1986.
- Jörn Bleck-Neuhaus: Elementary Particles. Modern physics from the atoms to the standard model . Springer, Heidelberg 2010, ISBN 978-3-540-85299-5 .
- David J. Griffiths: Introduction to Quantum Mechanics . 2nd Edition. Prentice Hall International, Upper Saddle River, NJ 2004, ISBN 0-13-111892-7 (Section 4.2 takes a closer look at the hydrogen atom).
- BH Bransden, Charles J. Joachain: Physics of Atoms and Molecules . Longman, London 1982, ISBN 0-582-44401-2 .
- Hagen Kleinert : Path Integrals in Quantum Mechanics, Statistics, Polymer Physics, and Financial Markets: 5th Edition . 5th edition. World Scientific Publishing Co Pte Ltd, 2009, ISBN 981-4273-55-4 (also readable online ).
Web links
Individual evidence
- ↑ More detailed and with other suggested models in: Helge Kragh Before Bohr: Theories of atomic structure 1850–1913. RePoSS: Research Publications on Science Studies 10. Århus: Department of Science Studies, University of Aarhus.
- ↑ see F. Dannemann: The natural sciences in their development and in their context , Vol. 3, Verlag W. Engelmann 1922, p. 198.
- ↑ JJ Balmer: Note on the spectral lines of hydrogen , Annalen der Physik Vol. 25 (1885) pp. 80-87 (online at wiley: Vol. 261 Issue 5).
- ↑ JJ Thomson: Phil. Mag. Vol. 44 (1897) p. 547.
- ↑ JJ Thomson: Phil. Mag. Vol. 6 (1903) p. 673.
- ^ AE Haas: Phys. Magazine Vol. 11 (1910) p. 537.
- ^ W Pauli : About the hydrogen spectrum from the standpoint of the new quantum mechanics . In: Journal of Physics . 36, 1926, pp. 336-363. doi : 10.1007 / BF01450175 .
- ^ Hagen Kleinert : Group Dynamics of the Hydrogen Atom . In: Lectures in Theoretical Physics, edited by WE Brittin and AO Barut, Gordon and Breach, NY 1968 . 1968, pp. 427-482.
- ^ Duru IH, Hagen Kleinert : Solution of the path integral for the H-atom . In: Physics Letters B . 84, No. 2, 1979, pp. 185-188. doi : 10.1016 / 0370-2693 (79) 90280-6 .
- ↑ Duru IH, Hagen Kleinert : Quantum Mechanics of H-Atom from Path Integrals . In: Fortschr. Phys . 30, No. 2, 1982, pp. 401-435. doi : 10.1002 / prop.19820300802 .