Xylans
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
| General | |||||||
| Surname | Xylan | ||||||
| CAS number | 9014-63-5 | ||||||
| Monomers / partial structures | Xylose | ||||||
| Type of polymer | |||||||
| Brief description |
light beige powder |
||||||
| properties | |||||||
| Physical state |
firmly |
||||||
| Melting point |
> 300 ° C |
||||||
| solubility |
insoluble in water |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
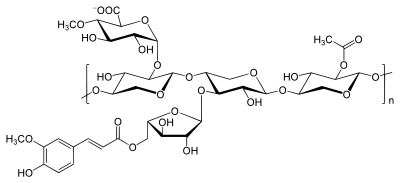
Xylans are vegetable heteropolysaccharides and belong to the hemicelluloses , their main repeating unit ( monomer unit ) is D - xylose . In nature, xylans are the second most common polysaccharide. Because of the different possibilities for modification, xylans are not clearly definable components.
Xylans as polysaccharides from plants
Xylans were given this name by EW Allen and Bernhard Tollens around 1890 because they assumed that xylose was the only hydrolysis product of these natural substances . Along with cellulose, they are probably the most important polysaccharide in the plant world. Xylans are the most important representatives of the hemicelluloses or polyoses.
The functions and structural properties of the xylans can be best demonstrated in comparison with the cellulose. In contrast to cellulose, they are not based on a single monomer that is polymerized to different degrees . Rather, they differ in their proportions and their chemical structure depending on the plant families . According to their monosaccharide , uronic acid and acetyl building blocks, a broad distinction is made between arabino-4- O -methylglucuronoxylan from conifers and, in a more diverse form, from monocotyledons and O -acetyl-4- O -methylglucuronoxylan from hardwoods , as well as some other, less common structures and subsequent modifications e.g. B. by digestion process . As monosaccharides D - xylose , D - mannose , D - glucose , D - arabinose , D - galactose , D - glucuronic acid and D - galacturonic act.
Another difference to cellulose is the much shorter chain length of the xylans, which in lignocelluloses is normally a maximum of 200 building blocks.
The side groups of xylans do not allow crystalline structures to build up to the same extent as in cellulose, so that xylan is only amorphous in vivo , although the formation of mixed crystals with cellulose is also being considered. The tasks of xylan as hemicellulose in the framework of structural materials also differ from cellulose. In the case of hemicelluloses, it is not the strength properties that are in the foreground, rather they act as an intermediary between cellulose and lignin . Their function is therefore also described as that of a natural plasticizer and support material. For the substance or the mixture of substances (mainly xylans) that can be extracted from wood by dilute lye, the term wood rubber was previously used.
To show the distribution of the hemicelluloses and thus also the xylan in the cell wall composite, a number of different models have been developed which have in common that the hemicelluloses form a connecting layer around the cellulose fibrils , which allows the cellulose to be embedded in the lignin matrix. This is indicated by the absence of hemicelluloses in lignin-free natural cellulose such as cotton . Another reason for the presence of hemicelluloses would be to look for their hygroscopic property, which could contribute to a moisture balance in the cell wall.
The totality of the different chemical and physical structural features of the xylans, which are influenced not only by their origin but also by isolation methods and technical processes, form a very complex system. The ultimate properties and, if applicable, the suitability for the intended use, be it in cellulose or in isolated form, can only be obtained from the interaction of all these points.
Occurrence of xylans
As already mentioned, xylans are the most widespread hemicelluloses in nature, they are found in all land plants and also in some algae . In coniferous and hardwood, they each make up about 10–15% and 10–35% of the wood substance. The range of these figures shows, however, that there are significant differences in the levels between different species, in different tissues and in the different layers of the cell wall. According to the required task in the plant, the xylan content z. B. on the different cell wall layers. More xylan can be found in the tracheids of latewood than in those of earlywood .
Since xylans are also contained in high proportions (up to 40%) in the biomass of monocotyledons and thus also in agricultural waste products, they are at least theoretically available in enormous amounts as raw material .
Average degree of polymerisation (DP)
For the degree of polymerization (Pn) of wood xylans, average values are found for arabino-4-O-methylglucuronoxylan of greater than 120 and for O-acetyl-4-O-methylglucuronoxylan of 200. However, there is a wide range of fluctuations, which depends on the type of wood considered . The literature values for the coniferous xylans range from 73 to 185 and for the hardwood xylans from 86 to 218. The degrees of polymerization can be significantly higher in monocotyledons, for water-soluble xylan from rye bran there are DP values of 418, for the corresponding water-insoluble fraction even 674. However the values obtained are influenced to a large extent by the insulation and measurement methods used and are therefore subject to large fluctuations.
Main and side chains


The linkage of the main chain , which always only consists of β-D-xylopyranose units (xyl), is possible either in β- (1 → 3) or β- (1 → 4) glycosidic form, whereby the β- ( 1 → 3) form only algae and seaweeds was found. Normally, deciduous and softwood xylans are assumed to have unbranched chains, but one or two branches could also occur per molecule.
In addition to the possible branches, side groups of sugars and sugar derivatives occur to varying degrees on the basic chain. While Esparto-Xylan only has side chains from further xylose units, i.e. real branches, one finds the most complex xylans v. a. in special tissues (e.g. seed coats ) of monocotyledons. The branches mostly only consist of monomeric units, but can also be dimeric or oligomeric . L - arabinofuranose (Ara), D - xylopyranose and, in rare cases, hexoses are used as sugar components . Rhamnose is found in small amounts as part of the end group in the xylan. Other possible side chains are formed by the uronic acids D- glucuronic acid (GluA) and 4- O -methyl- D- glucuronic acid (Me-GluA), as well as O -acetyl groups (Ac).
As mentioned above this is O -acetyl-4-O-Methylglucuronoxylan typical of the hardwoods and arabino-4- O typical -Methylglucuronoxylan for softwoods. But arabinoxylans have also been isolated from hardwoods, e.g. B. from laurel plants or cinnamon bark .
The 4- O -methylglucuronic acid side chains are linked α- (1 → 2) -glycosidically on carbon atom 2 of the xylose ring, the arabinofuranose building blocks of the coniferous and monocotyledon xylanes α- (1 → 3) -glycosidically on carbon atom 3 of the xylose. In hardwood xylans (the tempered zone), 50% to 60% of the xylose units also carry O -acetyl groups, but the total content can vary from approx. 3% to approx. 12% of the mass. In birch xylan, the ratio of unsubstituted , C2, C3 and di- substituted building blocks is 44: 24: 22: 10. A high degree of acetylation of the xylan is beneficial for water solubility . In wood, the degree of acetylation decreases with age, since v. a. Forms acetic acid in the heartwood through an acidic medium from the acetyl side groups .
In addition to the frequency of side groups, the composition of the side groups and their distribution along the chain are very important for the properties of the xylans, especially their solubility , since intermolecular aggregations can be prevented by the “disturbances” that the substituents represent .
For hardwood xylan the most frequently found average ratio of xylose to 4- O- methyl-glucuronic acid is 10: 1. The range is 6: 1 to 11: 1, with fractions with ratios of 3: 1 also being found. The distribution of the side chains within the xylan of a plant is therefore not constant.
The 4 O -methylglucuronic acid proportions are higher for conifers . There the ratio of xylan to 4- O -methyl-glucuronic acid is usually around 5-6: 1, with values of 3 to 4: 1 also occurring. The arabinose groups occur on the xylan in a ratio of (xyl: ara) 6-10: 1 on average, and thus also show great variability. The average ratio of the three components in softwood can be given as 8: 1.6: 1 (Xyl: Me-GluA: Ara), with the values fluctuating between 10: 3: 1 and 2.5: 0.8: 1. As could be shown on various softwoods and hardwoods, the distribution of the 4- O -methylglucuronic acid residues along the chain is different in the two groups of wood species . While in softwood xylan there is a more or less even distribution of the side groups across the xylan basic chain at every 7th to 8th xylose unit, in hardwood xylan areas with intensive substitution (about every second xylose unit) and those without side groups alternate irregularly. Due to the many hydroxyl groups available in hemicelluloses and the amorphous structure, they show a strong hygroscopicity and are therefore largely responsible for the swelling and shrinking of lignocellulose materials due to their embedding in the cell wall structure.
Biodegradation
In nature, xylans are broken down by so-called xylanases . These enzymes catalyze the hydrolysis of the main and side chains. Xylanases have been found in marine algae, protozoa , crustaceans , insects , snails, and seeds of land plants. Mainly the degradation of microorganisms takes place, so that filamentous fungi are mainly responsible for the biological degradation. The proportion of secreted xylanases is higher than in other microorganisms such as yeast or bacteria.
Because of the inhomogeneous structure of xylans, the degradation is not carried out by a xylanase but by an enzyme complex. Endoxylanases ( EC 3.2.1.8 ) and β- D -xylosidases ( EC 3.2.1.37 ) are particularly important here. To cleave the modified side radicals, also be ferulic acid - esterases , coumaric acid esterases, acetyl xylan esterases ( EC 3.1.1.6 ), α-glucuronidases and arabinases ( EC 3.2.1.99 ) were used.
Individual evidence
- ↑ a b c d e Data Xylane (PDF) at Carl Roth , accessed on 14 December of 2010.
- ↑ a b c d e Polizeli, ML. et al . (2005): Xylanases from fungi: properties and industrial applications . In: Appl Microbiol Biotechnol . 67 (5); 577-591; PMID 15944805 ; doi : 10.1007 / s00253-005-1904-7 .
literature
- Polizeli, ML. et al . (2005): Xylanases from fungi: properties and industrial applications . In: Appl Microbiol Biotechnol . 67 (5); 577-591; PMID 15944805 ; doi : 10.1007 / s00253-005-1904-7
- York, WS. and O'Neill, MA. (2008): Biochemical control of xylan biosynthesis - which end is up ? In: Curr Opin Plant Biol . 11 (3); 258-265; PMID 18374624 ; doi : 10.1016 / j.pbi.2008.02.007
- BY. Wang: Environmental biodegradation research focus . New York Nova Science Publishers 2007; ISBN 9781600219047
Web links
- Scaffolding materials TU-Darmstadt ( Memento from May 27, 2005 in the Internet Archive ) ( PDF , 164 KB)
