Zinc pyrithione
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
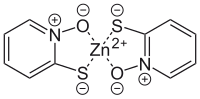
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Pyrithione zinc | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 8 N 2 O 2 S 2 Zn | |||||||||||||||
| Brief description |
an almost white powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 317.72 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
240 ° C (decomposition) |
|||||||||||||||
| solubility |
almost insoluble in water, slightly soluble in many organic solvents |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Zinc pyrithione ( INN ) is the zinc salt of pyrithione (pyridine-2-thiol-1-oxide). It is a colorless solid with antifungal and antibacterial properties. Known by several chemical and trade names, it was first described in the 1930s, and is used extensively, primarily in industry and cosmetics.
presentation
Zinc pyrithione is prepared starting from 2-chloropyridine . The largest producer of zinc pyrithione is Arch Chemicals ( Lonza Group ).
properties
Zinc pyrithione is chemically incompatible with paints that rely on metal carboxylate to cure. When used in water-based acrylic paint, a binding agent for the iron ions is required if there is a high iron content in the water. Zinc pyrithione is only slowly decomposed by ultraviolet radiation , which means that it can withstand direct sunlight for years. This and the so far only partially understood biological effects , which extend to the genetics and metabolism of many plant and animal cell types, represent a desired effect or an undesirable side effect, depending on the situation .
use
medicine
Zinc pyrithione is best known for its use against excessive dander and fungus. The medical diagnoses include seborrheic and other eczema , psoriasis , athlete's foot and other forms of tinea .
Biocide
Zinc pyrithione has a biocidal effect:
Due to its low solubility in water (8 ppm at neutral pH value), zinc pyrithione is suitable for use in exterior paints and other products that offer protection against mold . The fungus-inhibiting effect seems to stem from its ability to interrupt the transport of substances across the cell membrane by blocking the proton pump that nourishes the transport mechanism. Fungi are able to inactivate pyrithiones in low concentrations. Zinc pyrithione is also an effective algicide .
Zinc pyrithione is also used for the antibacterial treatment of sponges (especially from 3M ; brand: Scotch).
unwanted effects
The antibacterial properties of zinc pyrithione against pathogens from the streptococci and staphylococci family advertised by some suppliers are to be seen with the well-known caveat that applies to all external applications of this kind: there is a reduction in physiological colonization, the development of resistance with the promotion of MRSA and to impair the acid mantle of the skin.
While the EU had declared zinc pyrithione to be safe for human, veterinary and environmental medicine in a classification from 2002, the US EPA warned in an assessment from 2004 that zinc pyrithione is a strong poison even in the lowest concentration (ppb) on fish, invertebrates and aquatic plants in fresh and salt water. It is possible that this compound, acting as an endocrine disruptor, has hormone-like side effects, so there is a need for further research. In view of the use of the compound as a growth-inhibiting protective coating in shipbuilding, these concerns were repeated.
In primary skin cell culture zinc pyrithione induces a massive expression of heat shock proteins - encoding genes in keratinocytes and melanocytes , resulting in poly (ADP-ribose) polymerase 1 -abhängigem ATP can lead deficiency and necrotic cell death. Even in the nanomolar concentration range, the compound reduces the genetic integrity of important cells of the immune system (thymocytes, lymphocytes) in cell cultures of mice and humans.
Web links
- Entry for Zinc pyrithione in the Consumer Product Information Database
Individual evidence
- ↑ Entry on ZINC PYRITHIONE in the CosIng database of the EU Commission, accessed on March 11, 2020.
- ↑ a b c Entry on zinc pyrithione. In: Römpp Online . Georg Thieme Verlag, accessed on December 28, 2014.
- ↑ a b Entry on zinc pyrithione in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ Entry on zinc pyrithione in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ What is Skin Zinc? . Retrieved August 24, 2007.
- ^ CW Jones: Applications of Hydrogen Peroxide and Derivatives . Royal Society of Chemistry, 2007, ISBN 978-1-84755-013-2 ( Google Books ).
- ↑ Arch Chemicals Expanding Global Biocides Manufacturing Capacity , March 19 of 2008.
- ↑ Zinc Omadine® 48% FPS and Zinc Omadine® Enhanced CP Dispersion for Personal Cleansing
- ↑ CJ Chandler, IH Segel: Mechanism of the antimicrobial action of pyrithione: effects on membrane transport, ATP levels, and protein synthesis . In: Antimicrob. Agents Chemother. . 14, No. 1, 1978, pp. 60-68. PMID 28693 . PMC 352405 (free full text).
- ↑ ( Page no longer available , search in web archives: Notice of Filing a Pesticide Petition to Establish )
- ↑ Preliminary study on fouling protection for seagoing ships, part 2 ( Memento from June 10, 2007 in the Internet Archive ) (PDF; 447 kB), accessed on May 29, 2009
- ↑ SD Lamore, CM Cabello, GT Wondrak: The topical antimicrobial zinc pyrithione is a heat shock response inducer that causes DNA damage and PARP-dependent energy crisis in human skin cells. In: Cell Stress Chaperones. 15, 2010, pp. 309-322, PMID 19809895 , PMC 286699 (free full text).
- ^ JJ Mann, PJ Fraker: Zinc pyrithione induces apoptosis and increases expression of Bim. In: Apoptosis. 10, 1996, pp. 369-379, doi: 10.1007 / s10495-005-0811-9 .