Dermatophytosis
| Classification according to ICD-10 | |
|---|---|
| B35 | Dermatophytosis (tinea) |
| B35.0 | Tinea barbae and tinea capitis |
| B35.1 | Tinea unguium |
| B35.2 | Tinea manuum |
| B35.3 | Tinea pedis |
| B35.4 | Tinea corporis |
| B35.5 | Tinea imbricata |
| B35.6 | Tinea cruris |
| B35.7 | Other dermatophytoses |
| B35.8 | Dermatophytosis, unspecified |
| ICD-10 online (WHO version 2019) | |
The Dermatophytosis ( synonym Dermatophytie , from ancient Greek τὸ δέρμα derma , German 'skin and ancient Greek φυτόν phyton , German , Plant' ) or tinea ( Latin for, woodworm ',' moth ') is by special fungi ( dermatophytes induced) fungal skin disease . It is restricted to keratin-containing structures such as the horny layer of the skin ( epidermomycosis ), hair ( trichophytia or trichomycosis ) or nails or claws ( nail fungus , onychomycosis ). Dermatophytoses are among the most common infectious diseases and occur worldwide.
Dermatophytes are adapted to certain main hosts, where often little or no inflammatory reactions are triggered. Diseases with main hosts are therefore usually milder, but more protracted than with other hosts. According to the main source of infection, a distinction is made mainly between humans ( anthropophilic ) and animals ( zoophilic ) transmitted pathogens. Since all zoophilic pathogens are also disease-causing for humans, the dermatophytoses they cause in animals represent diseases that can be transmitted to humans ( zoonoses ). Fungi that are transmitted through contact with the ground ( geophiles ) only play a subordinate role in practice Role.
The clinical appearance is very variable, usually reddening of the skin , increased flaking and blisters appear. The pathogen detection is complex and only reliable through a combination of several methods, but without it the diagnosis cannot be made with certainty. The treatment is usually carried out with antimycotics , accompanying hygienic measures should be taken.
Pathogen

Dermatophytes are hose fungi (Ascomycota) that colonize keratinized skin structures . A distinction is made according to the secondary fruit form ( anamorphic ) about 30 species, which are divided into the three genera Epidermophyton , Trichophyton and Microsporum . The literature provides different information on the number of disease-causing species, as the system is subject to changes. The main fruit forms ( teleomorphs ) of these pathogens are soil dwellers and are assigned to the genus Arthroderma , but such perfect (sexual) forms have not yet been proven for some dermatophytes. In contrast to other fungi ( yeasts , molds ), dermatophytes feed on keratin and carbohydrates . You can break down keratin using protein-splitting enzymes ( keratinases ).
Dermatophytes are adapted to certain main hosts, in which only minor inflammatory reactions are triggered, which ensures the survival of the fungi. Therefore, such infections are usually milder, but more protracted than those caused by non-adapted pathogens. The main hosts can even be asymptomatic carriers and represent the reservoir of pathogens . They are the main source of infection for other people or animals.
According to the main source of infection, a distinction is made mainly between humans ( anthropophilic ) and animals ( zoophilic ) transmitted pathogens. All zoophilic mushroom species can be transmitted from animals to humans, but also from human to human. This is why they had to be registered in Germany until 1981. The proportion of the respective fungi in human diseases varies. In the case of athlete's foot, anthropophilic pathogens dominate, whereas in dermatophytoses in the area of the scalp hair , zoophilic fungi are four times more common than anthropophilic ones. A transmission of anthropophilic pathogens to animals is also possible, albeit rarely. Although animals rarely get sick with anthropophilic dermatophytes, they are a potential reservoir for some of these pathogens. In addition, there are also fungi that are transmitted through contact with the ground ( geophiles ), but these only play a subordinate role in practice.
The main triggers for dermatophytosis in Central Europe are Trichophyton rubrum (main host: humans), Trichophyton interdigitale (main host: humans), Trichophyton verrucosum (main host: cattle ) and Microsporum canis (main host: cats ). With regard to the frequency of the pathogen, however, there are historical and geographical differences, which are caused by the different living conditions. So dominated in humans in Germany before the Second World War still Microsporum audouinii and Epidermophyton floccosum . In southern Europe and the Middle East , most human dermatophytoses are caused by zoophilic pathogens such as Microsporum canis and Trichophyton verrucosum .
Occurrence

Dermatophytoses occur worldwide and are common, although in the vast majority of cases not life-threatening. They occur in mammals , birds and reptiles . The risk of developing dermatophytosis in the course of a person's life is around 10–20%. This makes dermatophytosis one of the most common infectious diseases . The incidence ( prevalence ) of athlete's foot in Germany is around 30% and that of nail fungus 12.4%. The prevalence in children is between 5 and 15%, the number of new cases ( incidence ) per year according to a Dutch study is around 25 per 1000 children.
In domestic cattle , around 40% of the herds in Germany are infected with Trichophyton verrucosum , clinical diseases ( bovine trichophytia ) occur in 5 to 60% of the animals within a herd. While the bovine trichophytic disease in Norway has been almost completely eradicated through consistent multi-year vaccination programs ( see section vaccination ), almost all herds in southern Europe are infected.
A study on mostly healthy domestic cats showed an infestation rate of 21.4%. About 90% of strays and practically all animals in cat breeding are mostly asymptomatic carriers. In an Austrian study, dermatophytes were found in 12.4% of domestic dogs . There is no epidemiological information on the occurrence of dermatophytes in rodents and rabbits kept in pets . Infestation frequencies between 1 and 70% were found in laboratory breeding. Asymptomatic carriers also dominate here, diseases occur mainly in guinea pigs .
Disease emergence

Infection occurs through direct or indirect contact with spores . The fungal spores are extremely resistant to environmental influences and infectious for up to four years. The zoophilic dermatophytes form (ectotric) spore layers around the hair , so that fungal hair is the main source of infection. The spores can also be transmitted by the wind and objects (combs, clothing, towels, blankets, floor mats, carpets ...).
For a fungal disease to occur, the spores have to penetrate the horny layer , nails or hair. In doing so, they have to overcome the mechanical barrier, the natural skin flora and the skin's immune system . Some dermatophytes produce β-lactam antibiotics and can damage the skin flora that protects the host. After the spores have attached, active penetration into the horny substance takes place through the formation of keratin- dissolving enzymes . The ability to produce various enzymes such as serine , aspartate and metalloproteases as well as hemolysins is a crucial virulence factor of dermatophytes. Then the thread-like fungal cells ( hyphae ) grow from the spores . Inflammation and mitotically active tissue prevent the development of the hyphae. Hair is only attacked in the growth phase ( anagen ), with the transition to the hair renewal phase ( telogen ) the infection comes to a standstill.

The extent of a dermatophytosis depends on the number of spores and their virulence as well as on host factors such as the immune system and the microclimate of the skin. According to virulence , a distinction is made between obligatory pathogenic dermatophytes, which generally cause disease, and facultative pathogenic dermatophytes, which only lead to disease under favorable circumstances. All zoophilic dermatophytes are obligatorily pathogenic for humans , so the diseases they cause are zoonoses . Dermatophytoses often break out due to the smallest skin injuries. Severe dermatophytoses occur almost exclusively in the event of defenses in the immune system as a result of immune deficiencies or the administration of drugs that suppress the immune system ( immunosuppressants ). Stress , other illnesses, misaligned feet, circulatory disorders , skin wrinkles as a result of being overweight , frequent contact with chemicals and a warm and humid climate are favorable factors.
Keratinocytes form interleukin-8 upon contact with dermatophyte antigens , which leads to an immigration of neutrophils , which have a cell-killing ( cytotoxic ) effect on dermatophytes. The organism reacts to the penetration of the spores with an immune response with the formation of antibodies ( IgM and IgG ). The cellular immune response is usually able to stop the disease. After the disease has healed, this immune response leads to unstable immunity , which does not protect the organism from a new disease, but in this case usually leads to faster healing. In the case of skin fungi that are adapted to the host, the immune reaction is usually weak, so that the clinical symptoms may be completely absent or only weakly pronounced ( latent infection ). It is believed that glycoproteins produced by the dermatophytes are able to weaken the host's immune response. On the other hand, especially with non-adapted hosts, there can also be an excessive immune response and thus allergic reactions of type I and IV (→ classification of allergies ). Allergic reactions to chronic dermatophytoses can presumably also contribute to the development of bronchial asthma . However, many cellular and molecular details in the course of defense reactions to dermatophytes have not yet been clarified.
Classification and clinical picture

Dermatophytosis can manifest itself in different ways, which can make diagnosis very difficult. Dermatophytes can attack the horny layer of the epidermis ( epidermomycosis ), hair ( trichophytia or trichomycosis ) and nails or claws ( nail fungus , tinea unguium or onychomycosis ).
In dermatology , dermatophytoses are further subdivided according to the location of their occurrence:
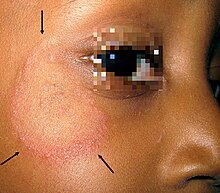
- Tinea capitis (from Latin caput ' head '), tinea in the area of the scalp hair , special form: Erbgrind ( Favus ),
- Tinea faciei (from Latin facies , face '),
- Tinea barbae (from Latin barba ' beard '),
- Tinea corporis (from Latin corpus ' body '), special form: Tinea imbricata (from Latin imbricatus 'roof tile-like'),
- Tinea inguinalis (from Latin inguen , groin '),
- Tinea manus (from Latin manus ' hand '),
- Tinea cruris (from Latin crus , lower leg ') and
- Tinea pedis (from Latin pes ' foot ').
In principle, neither the location of the occurrence nor the extent of the clinical changes can be used to draw any conclusions about the pathogen; athlete's foot can therefore be caused by various dermatophytes. In veterinary medicine, the classification according to the affected body region is not common. Another classification of dermatophytoses is according to the genus of the pathogen ( trichophytia , microsporia , epidermophytosis ), which, however, is not possible from the clinical picture, but requires a determination of the pathogen. Combinations of these classifications are also possible: Tinea capitis microsporica is a dermatophytosis in the area of the scalp hair caused by microsporum species.
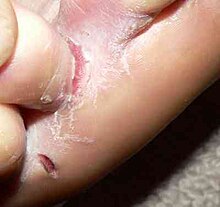
In the case of fungi that are adapted to a host, there are usually milder symptoms such as dull hair, focal (focal) hair breakage or falling out ("ringworm", "ringworm"), reddening , increased scaling or inflammation of the nail wall ( paronychia ). Itching is usually only mild. In the case of non-adapted fungal infections, excessive immune response, secondary bacterial infections or self-traumatization (scratching, licking), severe redness, vesicles and plaques , pus vesicles , pseudomycetomas or ulcerative changes ( kerion ) can occur, which are often accompanied by severe itching. In the case of a nail fungus, a crumbly, yellow-brownish discolored horn usually occurs. Very rarely, a life-threatening rose wound can result from a bacterial secondary infection .
Occasionally, as a hypersensitivity reaction to a skin fungus, an itchy skin reaction can occur in another place in which no dermatophytes can be detected, a dermatophytid .
Other fungal skin diseases , but also psoriasis , intertrigo , bacterial skin diseases (especially surface pyoderma ), eczema , lupus erythematosus and, in dogs, demodicosis can also be used for differential diagnosis .
Diagnosis
The diagnosis of dermatophytoses has been difficult and time-consuming up to now. None of the detection methods alone provided reliable results. The molecular detection of pathogen-specific gene segments opens up the possibility of safe and reliable diagnostics in the event of suspected dermatophytosis. The combination of different methods was imperative, the “gold standard” is still the combination of direct detection and fungal culture.
Direct detection of the pathogen
A simple and inexpensive investigation is the direct detection of the pathogens in the horn material. This is scraped off with a sterile wooden spatula or a ring curette . In the case of skin foci, this should be done from the edge area of the lesion, since vital fungi can only be expected there. If nail fungus is suspected, the horn material should be obtained from under the nail if possible; a ring curette is also used for this. If the hairy skin is infected with fungus, the hair is epilated with tweezers ; it is not enough to cut it off above the surface of the skin, as the dermatophytes only multiply in the hair follicle .

For further investigation, the horn material is covered with 15-20% potassium hydroxide (KOH) on a microscope slide in order to detach the fungi from the horn. The exposure time is about half an hour, the process can be accelerated by careful heating. To avoid crystallization, the sample should be stored in a moist chamber. Under a microscope , the let fungal hyphae and / or spores detected. If necessary, methylene blue can be added to the potassium hydroxide solution for staining the dermatophytes . The native detection with fluorescent dyes such as z. B. acridine orange under a fluorescence microscope .
The examination in the native material does not allow differentiation of the pathogen, and the differentiation of molds or yeasts from dermatophytes is difficult. In addition to artifacts make microscopic evaluation. For the detection of a nail fungus, however, this examination is more reliable than the fungal culture. About 15% of the examinations give false negative results.
The direct detection of gene segments of dermatophytes by means of PCR from samples is more reliable for the detection of dermatophytosis, but is not yet used in routine diagnostics. Due to the availability of a fully-fledged in-vitro diagnostic device (CE-IVD) for the simultaneous detection of 21 dermatomycosis pathogens in a multi-parameter approach, all the prerequisites for routine diagnostics are now in place.
Mushroom culture

Cultivation of the fungi from sample material on a nutrient medium (fungal culture) is advisable if there is any suspicion of dermatophytosis, also to identify asymptomatic carriers such as animals in the same household. Samples are taken in the same way as for direct detection; in the case of carrier animals with no symptoms, it is advisable to take skin flakes and hair using a soft toothbrush.
Kimmig agar and Sabouraud dextrose agar are mainly used for cultivation . In order to suppress the growth of other pathogens and unspecific microorganisms such as bacteria , yeasts or molds, inhibitors such as cycloheximide , gentamicin , chloramphenicol or chlortetracycline are used. The horn material is applied to the agar and incubated for at least two to three weeks. Only then have the dermatophyte cultures developed to such an extent that species differentiation is possible. The incubation usually takes place at 28–30 ° C, Trichophyton verrucosum grows best at an incubation temperature of 37 ° C. Many dermatophyte nutrient media have a phenol red additive as a pH value indicator, which leads to a red coloration of the nutrient medium due to the basic metabolic products of the dermatophytes. In contrast to molds or yeasts, dermatophytes first use the proteins in the nutrient medium and only then the carbohydrates . Therefore, the red color within the first week is an indication of dermatophytes, while it can also occur in the later incubation phase with molds and yeasts.
In addition to the appearance of the culture, the microscopic picture is also assessed. For this purpose, part of the culture is removed with a hook or with scotch tape and placed on a slide with a staining solution (e.g. methylene blue ). The arrangement of the micro- and macroconidia , the chlamydospores and hyphae allows conclusions to be drawn about the species. However, there are shape variations ( pleomorphism ) that can complicate species identification.
It should be noted that false-positive and false-negative results can always occur with a fungal culture. The sensitivity is only around 70%, i.e. almost a third of the fungal cultures give a false negative result. In the case of nail fungus samples, the sensitivity is only around 30%, since the fungi are often located in completely closed cavities. Therefore, additional cultures on special culture media and / or function tests ( urease detection, hair perforation test ), which are carried out in a laboratory specializing in mycology , may be necessary .
Skin biopsy
The punch biopsy followed by a histological examination is another diagnostic option. The sample should always be taken from the edge of the hearth. Histologically, an excessive increase in malformed horny cells in the horny layer (para hyperkeratosis ) dominates, as well as spongiosis in the epidermis and an inflammatory infiltrate in the dermis . The PAS reaction is usually used to better visualize the dermatophytes in the histological section . Histology can produce false negative results if the sample contains few or no spores. If immunological overreactions against the infection then take place, the histopathological picture can be misinterpreted.
Wood lamp
The Wood lamp - named after the American physicist Robert Williams Wood (1868–1955) - emits UV light in the range of 365 nm wavelength . This results in a positive result in a darkened room to a yellow-green fluorescence caused by tryptophan - metabolites caused the mushrooms in hair. When examining flakes of skin, crusts or fungal cultures, the fluorescence does not appear.
However, fluorescence only occurs in some types of dermatophyte: Microsporum canis (here only about half of the strains), and partly also in Microsporum distortum , Microsporum audouinii and Trichophyton schoenleinii . Negative fluorescence does not rule out dermatophytosis. False positive results can be caused by glowing crusts, lint or dust. The yeast Malassezia furfur (pathogen of Pityriasis versicolor ) and bacteria such as pseudomonads can also cause fluorescence. Another weak point of this investigation is that it is not possible to differentiate pathogens and thus also to determine the potential source of infection. The advantage of this method is the ability to initiate treatment immediately. Before doing this, however, samples should be taken for further examinations, because the therapy initiated may result in false negative results in the fungal culture.
Molecular Diagnostics
The immediate molecular diagnosis of dermatophytes made from native material from patients with suspected dermatomycosis using the polymerase chain reaction (PCR) is becoming increasingly important. The PCR duplicates pathogen-specific DNA sequences that are located in so-called marker gene areas of the respective dermatomycosis pathogens and allow a species-specific differentiation. The detection takes place z. B. by means of agarose gel electrophoresis with subsequent gel documentation. An easily manageable, routine and safe diagnosis of the most important dermatomycosis pathogens within a few hours is thus possible. The advantages of such a multi-parameter analysis include: a. the enormous time savings, the resulting therapeutic added value, the reliability of the methodology even with mixed samples and the ability to standardize the objective results. With the availability of an approved in-vitro diagnostic device (CE-IVD) for human diagnostics, all prerequisites have been created to utilize these advantages in the broad routine of laboratory medicine.
therapy

If the immune system is intact, dermatophytoses can heal spontaneously , i.e. without treatment, and this process can take several months. But they can also be chronic over several years. With a nail fungus , spontaneous healing is almost impossible. Mild forms of dermatophytosis of the foot and body can be brought about faster healing by using ointments or creams containing benzoic and salicylic acid . This combination of active ingredients was added to the World Health Organization 's list of essential medicines in 1977.
If a treatment is successful, a distinction must be made between clinical healing (disappearance of the skin changes) and mycological healing (complete elimination of the pathogen).
Antifungal drugs
The treatment of dermatophytosis with antimycotics always requires a reliable diagnosis, at least a positive native preparation should be available. Antimycotics are applied locally ( topically ) and / or systemically , depending on the type of disease . If the inflammation is severe , an anti-inflammatory glucocorticoid may be administered at the same time .
For topical treatment with ointments, creams and solutions, azoles such as clotrimazole , bifonazole , sertaconazole and miconazole , pyridones such as ciclopirox or allylamines such as terbinafine are used in particular . While most of the active ingredients are broad-spectrum antimycotics, i.e. they are also effective against other skin fungi, terbinafine has a more selective effect against dermatophytes. The local treatment of a nail fungus has been unsatisfactory for a long time, only newer active ingredients such as ciclopirox or amorolfine (both as nail polish containing active ingredients) show a better effect. The duration of treatment depends on the active ingredient and the severity of the disease and is between one week and six months. In Germany, only preparations based on miconazole and enilconazole are permitted for animals . Those unresponsive to therapy can in pet animals Human preparations rededicated , during in food-producing animals, the use of unapproved drugs for consumer protection reasons by the Regulation (EC) no. 470/2009 on maximum residue limits of pharmacologically active substances in foodstuffs of animal origin is strictly prohibited. In the Anglo-Saxon-speaking area , domestic treatment with diluted limescale has been common for several decades

Griseofulvin , terbinafine, itraconazole and fluconazole are used for systemic therapy . Griseofulvin is mainly used for dermatophytoses of the head and body and can be used in children after they are one year old. The other active ingredients are mainly administered to a nail fungus. The duration of treatment can be up to six months. Only itraconazole is approved for use in cats as a veterinary medicinal product.
Chitin inhibitors such as Lufenuron have no detectable effect on dermatophytoses.
vaccination
The development of vaccines from weakened dermatophytes or crude antigen fractions was discontinued in human medicine because of the triggering of autoimmune diseases . More recent research approaches are the use of recombinantly produced surface antigens from dermatophytes, which trigger a specific cellular immune response.
Several vaccines for the treatment of animals are approved in Germany for use in cattle, horses, dogs and cats. Vaccination in cats leads to faster clinical healing, especially in the case of initial infections and severe disease processes, but no mycological healing when used alone. Similar results were obtained in horses and guinea pigs. In Norway, the cattle population was rehabilitated by means of vaccinations, but this requires consistent vaccinations of the entire population over several years. The vaccination of foxes in fur farms was similarly successful .
Preventive and accompanying measures
Wearing protective shoes in public facilities (swimming pools, hotels, ...) is a preventative measure for athlete's foot. Since a warm, humid climate on the skin promotes the development of dermatophytes, thorough drying and wearing of open shoes are more effective preventive measures than, for example, disinfecting feet in a swimming pool. Items such as combs, brushes, clothes or towels should not be shared with other people.
A frequent change of linen and wearing kochbarer and welding good on sucking cotton clothes are recommended as accompanying measures. Affected areas of the skin should not be touched to prevent the spores from spreading to other parts of the body: Dermatophytosis on the hands is almost always the result of a foot or nail fungus.
In the case of long-haired animals in particular, shearing must be considered in order to reduce the amount of spores adhering to the hair. Consistent decontamination of the environment is usually indispensable in order to prevent renewed diseases (reinfections) in the case of zoophilic pathogens. Objects must be disinfected , washed with an effective disinfectant, cleaned with a steam jet or disposed of entirely. The choice of disinfectant is problematic. The fungi ( antifungal ), and sporicidal ( sporicidal ) activity is in the testing methods based on Candida albicans , a yeast so tested. However , a model developed on the basis of Microsporum canis showed that only dilute solutions of chlorine bleach , sulfur lime ( calcium polysulphide ) and enilconazole act safely against this dermatophyte. Of the quaternary ammonium compounds frequently used in disinfectants , only benzalkonium chloride showed a relatively good fungicidal effect.
history
Although dermatophytosis has occurred for many centuries, there are few reports of it. The term Favus ( Erbgrind ) was coined by Aulus Cornelius Celsus in the 1st century . In the 10th century, doctors like Avicenna and Rhazes described dry skin lesions which they called Sahafats and Alvathin . The term tinea was used for this in the Middle Ages . Celsus' work was first printed in German in 1561, and in 1690 Tobias Vogel wrote the first German dermatology book, in which various manifestations of dermatophytoses are described. Dermatophytoses were probably widespread in the Middle Ages. The painting Christ Carrying the Cross by Hieronymus Bosch (around 1450–1516) from 1510 shows a soldier with a corresponding skin change on his head and a painting by Rembrandt's pupil Ferdinand Bol (1616–1680) shows a boy who clearly suffers from hereditary grind. Even Goya (1746-1828) Images "boys when climbing a tree" and "The Wedding" ( La boda , 1792) represent head changes to a tinea capitis point.
The cause of the disease could only be clarified in the 19th century. The Berlin university professor Johann Lukas Schönlein (1793–1864) discovered in 1839 that hereditary grind is caused by fungi. Robert Remak (1815–1865) had discovered such structures four years earlier, but had not published his observations. But he had it cited in 1837 as a personal communication in the dissertation of his student Xaver Hube. Remak did not identify the spherical and rod-shaped structures as mushrooms, so he admitted the discovery to Schönlein and named the pathogen Achorion schoenleinii (today: Trichophyton schoenleinii ) after him . In addition, Remak recognized the infectiousness of the pathogen by infecting itself with spores and cultivated it for the first time on apple slices. Without knowing the work of Remak and Schönlein, the Hungarian doctor David Gruby (1810–1898) , who worked in Paris, researched clinical, epidemiological and mycological details about hereditary grind in the early 1840s, which is why he is also considered the founder of dermatomycology (skin fungus science). He also described the penetration of Microsporum audouinii from the outside into the hair (ectotrich) in tinea capitis and tinea barbae and the growth within the hair (endotrich) of Trichophyton tonsurans .
The French microbiologist Raymond Sabouraud (1864–1938) began to study dermatophytes intensively around 1890. He described their morphological features and established a taxonomy. The Sabouraud agar developed by him is still today, albeit in a slightly modified form, the standard nutrient medium for growing dermatophytes. He summarized his findings in 1910 in "Les Teignes", the first standard work on dermatophytoses. In 1925 Margarot and Deveze introduced the Wood lamp developed by Robert Williams Wood (1868–1955) in 1903 into mycological diagnostics.
The division of dermatophytes into three genera ( Epidermophyton , Trichophyton and Microsporum ), which is still common today, was established in 1934 by the American mycologist Chester Emmons (1900–1985) based on Sabouraud's work . The sexual forms ( teleomorphs ) of dermatophytes were discovered in 1959 by Dawson and Gentles in Trichophyton ajelloi , and in the 1960s also for other dermatophytes. The discovery of the sexual reproduction of dermatophytes was a prerequisite for genetic studies and research into their pleomorphism .
Dermatophytoses were treated with mercury , sulfur , thallium or iodine compounds until the beginning of the 20th century . From 1903 to the end of the 1950s, dermatophytosis patients were treated with X-rays . This led to an increased incidence of radiation-related cancers . The massive use of X-ray therapy among immigrants to Israel in the 1940s and 1950s was the subject of two documentary films and led to the passage of a compensation law in 1994 (→ ringworm affair ). In 1958, Gentles first used griseofulvin , which had already been isolated by Albert Edward Oxford and colleagues in 1939, in animal experiments on guinea pigs. This is considered to be the birth of antimycotic therapy. As early as November 1958, griseofulvin was successfully used in humans by the Viennese dermatologist Gustav Riehl . Attempts to develop dermatophyte vaccines began as early as the 1940s. Large-scale vaccinations were first carried out on domestic cattle in the Soviet Union in 1969 .
literature
- Otto Braun-Falco , Gerd Plewig , Helmut H. Wolff, Walter HC Burgdorf, Michael Landthaler: Dermatology and Venereology. 5th edition. Springer, Berlin a. a. 2005, ISBN 3-540-40525-9 .
- Peter Fritsch: Fungal diseases (mycoses). In: Peter Fritsch: Dermatology and Venereology. Textbook and atlas. Springer, Berlin a. a. 1998, ISBN 3-540-61169-X , pp. 282-302.
- Renate Hämmerling, Michael Cieslicki: Reliably diagnosing dermatophytoses and reducing the risk of zoonoses. In: Small Animal Practice. Vol. 54, No. 11, 2009, ISSN 0023-2076 , pp. 639-650, abstract .
- Chiara Noli, Fabia Scarampella: Dermatophytosis. In: Chiara Noli, Fabia Scarampella: Practical dermatology in dogs and cats. Clinic, diagnosis, therapy. Schlütersche, Hannover 2004, pp. 203-210, ISBN 3-87706-726-3 .
- Claus Seebacher: Dermatomycoses. Basics and therapy. Springer, Berlin a. a. 2000, ISBN 3-540-65100-4 .
- Hans-Jürgen Tietz, Horst Ulbricht: Human pathogenic fungi of the skin and mucous membranes. Collection, cultivation, differentiation. Schlütersche, Hannover 1999, ISBN 3-87706-540-6 .
- Irene Weitzman, Richard S. Summerbell: Dermatophytes. In: Clinical Microbiology Reviews. Vol. 8, No. 2, 1995, ISSN 0893-8512 , pp. 240-259, PMID 7621400 .
Web links
- German Dermatological Society: Guideline Tinea of Free Skin ( Memento from January 24, 2013 in the Internet Archive ) (PDF, 209 kB)
- German Dermatological Society: Tinea capitis guideline ( Memento from May 3, 2014 in the Internet Archive )
- Heinz Seeliger and Theresia Heymer: Diagnostics of pathogenic fungi in humans . Textbook and atlas. (PDF, 9.3 MB)
Individual evidence
- ↑ a b c P. Fritsch: Fungal diseases (mycoses) . In: Dermatology and Venereology. Textbook and atlas . Springer-Verlag, Berlin, Heidelberg 1998, ISBN 3-540-61169-X , pp. 282-302.
- ↑ a b c d e f g h i j k l Renate Hämmerling and Michael Cieslicki: Reliably diagnose dermatophytoses and reduce the risk of zoonoses. In: Kleintierpraxis 54 (2011), pp. 639–650.
- ↑ a b c Irene Weitzman and Richard S. Summerbell: Dermatophytes . In: Clin. Microbiol. Rev. 8 (1995), pp. 240-259. PMID 7621400 , PMC 172857 (free full text)
- ↑ a b H.-J. Tietz and Renate Hämmerling: The importance of zoophilic dermatophytes for humans and anthropophilic zoonoses for animals. In: Prakt. Tierarzt 88 (2007), pp. 78-86.
- ↑ P. Van Rooij et al .: Canine dermatophytosis caused by Trichophyton rubrum: an example of man-to-dog transmission. In: Mycoses 2011 Jul 20. doi : 10.1111 / j.1439-0507.2011.02071.x . PMID 21771108
- ^ Regina Wagner: Dermatophytoses in dogs and cats. In: Kompendium Kleintier 2013, pp. 28–32.
- ↑ Claus Seebacher et al .: Updates on the epidemiology of dermatophyte infections. In: Mycopathologia 166 (2008), pp. 335-352. PMID 18478365
- ↑ LA Drake et al .: Guidelines of care for superficial mycotic infections of the skin: tinea corporis, tinea cruris, tinea faciei, tinea manuum, and tinea pedis. Guidelines / Outcomes Committee. American Academy of Dermatology. In: Journal of the American Academy of Dermatology . 34 (1996), pp. 282-286. PMID 8642094
- ↑ B. Havlickova et al .: Epidemiological trends in skin mycoses worldwide. In: Mycoses . 51 (2008), Suppl. 4, pp. 2-15. PMID 18783559
- ↑ Claus Seebacher: To change the dermatophyte spectrum in dermatologically relevant diseases. In: Mycoses 46 (2003), Suppl 1, pp. 42-46. PMID 12955853
- ↑ D. Abeck et al .: Onychomycosis: Current data on epidemiology, spectrum of pathogens, risk factors and effects on quality of life. In: Dtsch. Doctor bl. 97 (2000), pp. 1984-1986.
- ↑ RS Mohammedamin et al .: Reported incidence and treatment of dermatophytosis in children in general practice: a comparison between 1987 and 2001. In: Mycopathologia 164 (2007), pp. 271-278. PMID 17891509 . PMC 2780650 (free full text)
- ↑ HH Zehle et al .: To combat trichophytia. In: 58th Veterinary Exchange of Experience, May 23, 2007. Full text ( Memento from January 16, 2014 in the Internet Archive )
- ^ R. Papini et al .: High infection rate of Trichophyton verrucosum in calves from Central Italy. In: Zoonoses and Public Health . 56 (2009), pp. 59-64. PMID 18705659
- ↑ KH Böhm et al .: Preliminary results of current studies on the spread of the microsporia in cat populations. In: Tierärztl. Praxis 26 (1998), p. 73.
- ↑ KA Moriello: Management of dermatophyte infections in catteries and multiple-cat house holds. In: Vet. Clin. North Am. Small Anim. Pract. 25: 1457-1474 (1990). PMID 2251736
- ↑ R. Breuer Strosberg: frequency of detection of dermatophytes in cats and dogs in Austria . In: Dtsch. Veterinarian Weekly 100: 483-485 (1993). PMID 8306866
- ↑ A. Balsari et al .: Dermatophytes in clinically healthy laboratory animals. In: Lab. Anim. 15: 75-77 (1981). PMID 7265899 ( full text ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this note. , PDF, 745 kB)
- ↑ R. López-Martínez et al .: Dermatophytes isolated from laboratory animals. In: Mycopathologia, 88 : 111-113 (1984). PMID 6527696
- ^ C. Pollock: Fungal diseases of laboratory rodents. In: Vet. Clin. North Am. Exotic. Anim. Pract. 6 (2003), pp. 401-413. PMID 12827729
- ↑ KA Moriello and DJ DeBoer: Feline dermatophytosis. Recent advances and recommendations for therapy. In: Vet. Clin. North Am. Small Anim. Pract. 20: 901-921 (1995). PMID 8525573
- ↑ a b c d e f g h Barbara Beifuß: Establishment of an immediate molecular diagnosis of dermatophytes at the species level from native material from patients with skin mycoses using polymerase chain reaction (PCR) and real-time PCR (LightCycler). Diss. Ludwig Maximilians University Munich 2008. ( Full text , PDF, 1.5 MB)
- ↑ Ch. Noli and F. Scarampella: Dermatophytosis . In: Practical Dermatology in Dogs and Cats . Schlütersche, Hannover 2004, pp. 203-210, ISBN 3-87706-726-3
- ↑ P. Hensel: The feline dermatophytosis - diagnosis and therapy . In: Kleintiermedizin (5/6) / 2006, pp. 122–132
- ↑ a b c S.R. Almeida: Immunology of dermatophytosis. In: Mycopathologia 166 (2008), pp. 277-283. PMID 18478362 .
- ↑ JA Woodfolk: Allergy and dermatophytes. In: Clin Microbiol Rev. 18 (2005), pp. 30-43. PMID 15653817 . PMC 544172 (free full text)
- ↑ a b c d e f g Otto Braun-Falco and Helmut Heinrich Wolff: Dermatology and Venereology . Springer, 5th edition 2005, ISBN 9783540405252 .
- ↑ Michael Rolle and Anton Mayr: Medical microbiology, infection and epidemic theory . Georg Thieme Verlag, 7th edition 2007, ISBN 9783830410607 .
- ↑ JC Roujeau et al .: Chronic dermatomycoses of the foot as risk factors for acute bacterial cellulitis of the leg: a case-control study. In: Dermatology 209 (2004), pp. 301-307. PMID 15539893
- ↑ K.-H. Huhnstock, W. Kutscha and H. Dehmel: Diagnosis and therapy in practice . Springer-Verlag, 5th ed. 2013, ISBN 9783642683855 , pp. 85–86.
- ↑ V. Panasiti et al .: Comparison of diagnostic methods in the diagnosis of dermatomycoses and onychomycoses. In: Mycoses 49 (2006), pp. 26-29. PMID 16367815
- ↑ a b H.-J. Tietz and H. Ulbricht: Human pathogenic fungi of the skin and mucous membranes . Schlütersche Hannover 1999, ISBN 3-87706-540-6
- ↑ D. Wilsmann-Theis et al .: New reasons for histopathological nail-clipping examination in the diagnosis of onychomycosis. In: Journal of the European Academy of Dermatology and Venereology . 25 (2011), pp. 235-237. PMID 20477921
- ↑ J. Garg et al .: Rapid detection of dermatophytes from skin and hair. In: BMC Res Notes. 18 (2009), p. 60. PMID 19374765 . PMC 2678142 (free full text)
- ↑ A. Hryncewicz-Gwóźdź et al .: Identification and differentiation of Trichophyton rubrum clinical isolates using PCR-RFLP and RAPD methods. In: Eur J Clin Microbiol Infect Dis . 30 (2011), pp. 727-731. PMID 21416216 PMC 3088811 (free full text)
- ↑ Press release: Well-being is a matter of the SKIN ( full text )
- ^ MA Lawry et al .: Methods for diagnosing onychomycosis: a comparative study and review of the literature. In: Archives of Dermatology . 136 (2000), pp. 1112-1116. PMID 10987866
- ↑ Renate Hämmerling: Dermatophytosis in a dog with unusual symptoms. In: Veterinärspiegel 2 (2004), pp. 78–82.
- ^ LK Gupta and MK Singhi: Wood's lamp. In: Indian Journal of Dermatology, Venereology and Leprology . 70 (2004), pp. 131-135. ( Full text )
- ↑ Claus Seebacher: Dermatomycoses: Basics and Therapy . Springer-Verlag 2000, ISBN 9783540651000
- ↑ WHO Model List of Essential Medicines (PDF, 442 kB), accessed on September 20, 2012.
- ↑ a b c d Monika Linek: Update of the therapy of dermatophytoses. In: Kleintierpraxis 54 (2009), pp. 622–632.
- ↑ Veterinary dermatological study from 2011: A. Diesel, M. Verbrugge, KA Moriello: Efficacy of eight commercial formulations of lime sulfur on in vitro growth inhibition of Microsporum canis. In: Veterinary Dermatology . Volume 22, Number 2, April 2011, ISSN 1365-3164 , pp. 197-201, doi : 10.1111 / j.1365-3164.2010.00928.x , PMID 20868396 . See in particular the introductory sentence "Lime sulfur is a common topical treatment for dermatophytosis in animals".
- ↑ Michael Cieslicki: Clinical and mycological effectiveness of Lufenuron in the dermatophytosis of the cat . In: Kleintierpraxis 9 (2005), pp. 575-580.
- ↑ a b Doris Westhoff et al .: The treatment of feline dermatophytosis with an inactivated fungal vaccine . In: Kleintierpraxis 8 (2011), pp. 401–410.
- ↑ J. Karle et al .: Controlled blind study to prove the efficacy of the inactivated vaccine Insol Dermatophyton in artificially infected horses. In: Pferdeheilkunde 18 (2002), pp. 625–628.
- ^ Z. Hussin and JM Smith: Vaccination procedures and the infectivity of dermatophyte lesions. In: Mycopathol . 40, pp. 71-76 (1983). PMID 6855874
- ↑ a b R. Gudding and A. Lund: Immunoprophylaxis of bovine dermatophytosis. In: Can Vet J . 36 (1995), pp. 302-306. PMID 7773918 , PMC 1686876 (free full text)
- ↑ LK Bredahl et al .: Efficacy of an experimental Microsporum canis vaccine in farmed foxes. In: Veterinary Dermatology . 12 (2000), p. 45.
- ↑ a b Mark Buchta et al .: The second StEx: Basic knowledge of clinical medicine for exams and practice . Springer-Verlag, 2nd edition 2004, ISBN 9783540203513 , p. 388.
- ↑ Renate Hämmerling: Dermatophytoses in the small animal practice. In: Kleintiermedizin 7 (2004), pp. 1–6.
- ↑ KA Moriello et al .: Development of an in vitro, isolated, infected spore testing model for disinfactant testing of Microsporum canis isolates. In: Veterinary Dermatology . 15 (2003), pp. 175-179. PMID 15214954
- ^ A b c R. Negroni: Historical aspects of dermatomycoses. In: Clinics in Dermatology . 28 (2010), pp. 125-132. PMID 20347652
- ↑ a b Hannelore Mittag: The tinea from a historical point of view . In: 15th conference of the working group “Mykologische Laboratoriumsdiagnostik” within the German-speaking Mykological Society (DMykG), Leipzig, November 16, 2001 ( full text ( memento of the original from March 9, 2013 in the Internet Archive ) Info: The archive link was automatically inserted and still not checked. Please check the original and archive link according to the instructions and then remove this note. , PDF, 349 kB)
- ↑ 50 years of the German-speaking Mycological Society ( Memento of the original from September 29, 2011 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF, 389 kB)
- Jump up ↑ J. Margarot and P. Deveze: Aspect de quelques dermatoses lumiere ultraparaviolette. Note preliminaire. In: Bull. Soc. Sci. Med. Biol. Montpellier 6 (1925), pp. 375-378.
- ↑ CO Dawson and J. Gentles: Perfect stage of Keratinomyces ajelloi. In: Nature 183 (1959), pp. 1345-1346.
- ^ RE Shore et al .: Skin Cancer after X-Ray Treatment for Scalp Ringworm . In: Radiation Research 157 (2002), pp 410-418. PMID 11893243
- ↑ J. Gentles: experimental ringworm in guinea pigs: oral treatment with griseofulvin. In: Nature 182 (1958), pp. 476-477.
- ^ AE Oxford et al .: Studies in the biochemistry of microorganisms: Griseofulvin, C (17) H (17) O (6) Cl, a metabolic product of Penicillium griseofulvum Dierckx. In: Biochemical Journal . 33 (1939), pp. 240-248
- ↑ Gustav Riehl: Griseofulvin as an orally effective antimycotic. In: Dermacol. Wochenschrift . 140 (1959), p. 993.