1-chlorobutane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 1-chlorobutane | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 9 Cl | |||||||||||||||
| Brief description |
highly volatile, colorless liquid with a chloroform- like odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 92.57 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.89 g cm −3 |
|||||||||||||||
| Melting point |
−123 ° C |
|||||||||||||||
| boiling point |
78 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
very bad in water (0.87 g l −1 at 25 ° C) |
|||||||||||||||
| Refractive index |
1.4018 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
1-chlorobutane is a chemical compound from the groups of saturated, aliphatic halogenated hydrocarbons and organic chlorine compounds .
synthesis
A technical synthesis takes place through the thermal chlorination of butane over aluminum oxide at 200 ° C. This produces 2-chlorobutane to the same extent and small proportions of dichlorobutane . This synthesis is also successful photochemically at 15-20 ° C, the product distribution being similar. Another possibility is the reaction of 1-butanol with hydrogen chloride at 100 ° C.
properties
1-chlorobutane is a colorless and highly volatile liquid with a typical odor of halogenated hydrocarbons. The compound boils at 78 ° C. under normal pressure. At −123.1 ° C the substance solidifies to a colorless solid. 1-chlorobutane forms azeotropically boiling mixtures with water and alcohols . With a water content of 6.6 % by mass, an azeotrope appears at 68.1 ° C. The azeotropes for alcohols are for methanol with 28.5 mass% at 57.2 ° C, for ethanol with 21.5 mass% at 66.2 ° C and for 1-propanol with 16.0 mass% at 75.6 ° C.
Thermodynamic properties
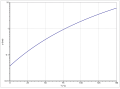
According to Antoine, the vapor pressure function results from log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 3.99588, B = 1182.903 and C = −54.885 in the temperature range from 256.4 to 351.6 K.
| property | Type | Value [unit] | Remarks |
|---|---|---|---|
| Standard enthalpy of formation | Δ f H 0 liquid Δ f H 0 gas |
−188.2 kJ mol −1 −154.6 kJ mol −1 |
|
| Enthalpy of combustion | Δ c H 0 liquid | −2695.8 kJ mol −1 | |
| Heat capacity | c p | 159.53 J mol −1 K −1 (25 ° C) 1.72 J g −1 K −1 (25 ° C) |
as a liquid |
| Critical temperature | T c | 503 K | |
| Critical pressure | p c | 45.8 bar | |
| Critical volume | V c | 0.247 l mol −1 | |
| Critical density | ρ c | 0.318 g ml −1 | |
| Acentric factor | ω c | 0.228 | |
| Enthalpy of evaporation | Δ V H 0 Δ V H |
33.52 kJ mol −1 30.39 kJ mol −1 |
at normal pressure boiling point |
The temperature dependence of the enthalpy of vaporization can be calculated according to the simplified Watson equation Δ V H = A · (1 − T r ) n (Δ V H in kJ / mol, T r = (T / T c ) reduced temperature) with A = 41.205 kJ / mol, n = 0.336 and T c = 532.0 K in the temperature range between 184 K and 532 K.
Vapor pressure function of 1-chlorobutane
Safety-related parameters
1-chlorobutane is considered a flammable liquid. Flammable vapor-air mixtures can form above the flash point. The compound has a flash point of −12 ° C. The explosion range is between 1.8% by volume (65 g / m 3 ) as the lower explosion limit (LEL) and 10.1% by volume (390 g / m 3 ) as the upper explosion limit (UEL). The maximum explosion pressure is 9.6 bar. The limit gap width was determined to be 1.06 mm. This results in an assignment to explosion group IIA. With a minimum ignition energy of 1.24 mJ, vapor-air mixtures are extremely ignitable. The ignition temperature is 245 ° C. The substance therefore falls into temperature class T3.
use
1-chlorobutane is used as an alkylating reagent to introduce a butyl group in organic syntheses (e.g. for ionic liquids ). Reaction with metallic lithium gives butyllithium , which is very often used for organometallic syntheses .
The compound has very good dissolving properties for fats , oils and waxes and is also used in HPLC . In veterinary medicine 1-chlorobutane is used as an agent against lower worms .
Individual evidence
- ↑ a b c d e f g h i j k Entry on 1-chlorobutane in the GESTIS substance database of the IFA , accessed on March 10, 2018(JavaScript required) .
- ^ W. Gerrard, HR Hudson, WS Murphy: s-Butyl Chloride from n-Butyl Dichloroborinate and from n-Butanol-Hydrogen Chloride. In: J. Chem. Soc. 1962, pp. 1099-1101, doi: 10.1039 / JR9620001099 .
- ↑ Entry on 1-chlorobutane in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Data sheet 1-chlorobutane ( Memento of the original from March 25, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF; 91 kB) from Applicher (accessed August 27, 2012).
- ↑ a b c Ullmann's Encyclopedia of Industrial Chemistry. 7th edition. Wiley Interscience, Release 2009.
- ↑ a b c d e f g E. Brandes, W. Möller: Safety-related parameters. Volume 1: Flammable Liquids and Gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003.
- ↑ HR Kemme, SI Kreps: Vapor Pressure of Primary 'n'-Alkyl Chlorides and Alcohols. In: J. Chem. Eng. Data . 14, 1, 1969, pp. 98-102, doi: 10.1021 / je60040a011 .
- ↑ a b G. Stridth, p Sunner: Enthalpies of formation of some 1-chloroalkanes and the CH2-increment in the 1-chloroalkanes series. In: J. Chem. Thermodyn. 7, 1975, pp. 161-168, doi: 10.1016 / 0021-9614 (75) 90264-5 .
- ^ A b J. PE Grolier, G. Roux-Desgranges, M. Berkane, E. Jimenez, E. Wilhelm: Heat capacities and densities of mixtures of very polar substances 2. Mixtures containing N, N-dimethylformamide. In: J. Chem. Thermodyn. 25 (1), 1993, pp. 41-50, doi: 10.1006 / jcht.1993.1005 .
- ^ A b c d e Carl L. Yaws, Prasad K. Narasimhan: Thermophysical Properties of Chemicals and Hydrocarbons - Chapter 1: Critical Properties and Acentric Factor, Organic Compounds. 1st edition. Elsevier, 2008, ISBN 978-0-8155-1596-8 , p. 8, doi: 10.1016 / B978-081551596-8.50006-7 .
- ↑ V. Tekac, V. Majer, V. Svoboda, V. Hynek: Enthalpies of vaporization and cohesive energies for six monochlorinated alkanes. In: J. Chem. Thermodyn. 13, 1981, pp. 659-662, doi: 10.1016 / 0021-9614 (81) 90037-9 .
- ^ V. Majer, V. Svoboda: Enthalpies of Vaporization of Organic Compounds: A Critical Review and Data Compilation. Blackwell Scientific Publications, Oxford 1985, p. 300.
- ^ Carl L. Yaws, Marco A. Satyro: Thermophysical Properties of Chemicals and Hydrocarbons - Chapter 7: Enthalpy of Vaporation, Organic Compounds. 1st edition. Elsevier 2008, ISBN 978-0-8155-1596-8 , p. 315, doi: 10.1016 / B978-081551596-8.50012-2 .
- ↑ JB Fenn: Lean flammability limit and minimum spark ignition energy. In: Ind. Eng. Chem. 43, 1951, pp. 2865-2869, doi : 10.1021 / ie50504a057 .
- ↑ HF Calcote, CA Gregory, CM Barnett, RB Gilmer: Spark Ignition - Effect of Molecular Structure. In: Ind. Eng. Chem. 44, 1952, pp. 2656-2662, doi: 10.1021 / ie50515a048 .
- ↑ National Toxicology Program: n-Butyl chloride ( Memento March 5, 2016 in the Internet Archive ), accessed November 18, 2014.
Web links
Related links
- 2-chlorobutane
- tert -butyl chloride
- 1-chloroisobutane (1-chloro-2-methylpropane)
- 1-bromobutane