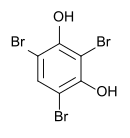
2,4,6-tribromoresorcinol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2,4,6-tribromoresorcinol | ||||||||||||||||||
| other names |
2,4,6-tribromobenzene-1,3-diol |
||||||||||||||||||
| Molecular formula | C 6 H 3 Br 3 O 2 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 346.8 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
114-116 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
2,4,6-tribromoresorcinol is a chemical compound that belongs to the group of phenols .
presentation
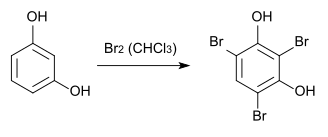
2,4,6-tribromoresorcinol can be made by bromination of resorcinol in chloroform .
If bromine is used in excess, the 2,4,6-tribromoresorcinol reacts further to 2,4,4,6,6-pentabromo-1-cyclohexene-3,5-dione. This reaction can be reversed by adding potassium iodide .
Reactions
In the laboratory, 2,4,6-tribromoresorcinol can be converted into 2-bromoresorcinol by reacting with sodium sulfite and sodium hydroxide in a 5: 1 water / methanol mixture .
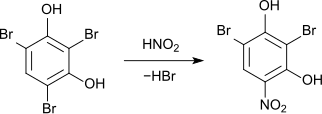
The nitration of 2,4,6-tribromo-resorcinol with nitrous acid leads to the displacement of a bromine atom with the formation of 2,6-dibromo-4-nitroresorcinol.
If the nitration is carried out with fuming nitric acid , two bromine atoms are displaced, resulting in 2-bromo- 4,6-dinitroresorcinol .
If the 2,4,6-tribromoresorcinol is esterified with acetic anhydride before nitration , the nitro group attaches itself to position 5. Also, the diethyl ether (m.p. 68-69 ° C) reacts in this way. The diacetate melts at 108 ° C.
The sodium salt of 2,4,6-tribromoresorcinol can be prepared in ethanolic solution and crystallizes with two molecules of ethanol with the stoichiometric composition C 6 HBr 3 (ONa) 2 · 2C 2 H 5 OH.
Individual evidence
- ↑ a b c data sheet 2,4,6-tribromoresorcinol from Sigma-Aldrich , accessed on March 17, 2011 ( PDF ).
- ↑ JELightowler, HJRylance: On the anti-inflammatory activity of some substituiertem phenolic compounds. In: British Journal of Pharmacology and Chemotherapy . 1964, Vol. 22, pp. 221ff, PMID 1703990 .
- ↑ a b Timo Liebig: Concave N-Heterocyclic Catalyst Systems . University of Kiel, 2006.
- ↑ Michael Abbass: Concave 1,10-phenantroline with additional functionality in the 4'-position. University of Kiel, 2002. urn : nbn: de: gbv: 8-diss-17594
- ↑ HP Latscha, HA Klein, GW Linti: Analytical Chemistry . 4th edition, Springer, 2003, ISBN 978-3-540-40291-6 , p. 287 ( limited preview in the Google book search).
- ↑ HH Hodgson, EW Smith: “The replacement of bromine in bromophenols by the nitro-group. Part I. 2: 4: 6-Tribromo-3-nitrophenol and -3-chlorophenol. Some cases of group migration” in J. Chem Soc. , 1931 , 2268-2272. doi : 10.1039 / JR9310002268
- ↑ a b c d e C. L. Jackson, FL Dunlap: Certain Bromine Derivatives of Resorcine. In: American Chemical Journal 1896, 18 , pp. 117ff. Full text