2-ethyl-2-oxazoline
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 2-ethyl-2-oxazoline | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 9 NO | |||||||||||||||
| Brief description |
colorless to pale yellow liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 99.13 g · mol -1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density | ||||||||||||||||
| Melting point |
−62 ° C |
|||||||||||||||
| boiling point |
|
|||||||||||||||
| Vapor pressure | ||||||||||||||||
| solubility |
miscible with water, ethanol and diethyl ether , soluble in acetonitrile , toluene , nitrobenzene and 1,2-dichloroethane |
|||||||||||||||
| Refractive index |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
2-Ethyl-2-oxazoline (EtOx) is a 2-oxazoline derivative and a cyclic imidic acid ester . The compound is used in particular as a monomer for cationic ring-opening polymerization to give poly (2-alkyloxazoline) s , which are being investigated as readily water-soluble and biocompatible materials for biomedical applications.
Manufacturing
Made from propionic acid and its derivatives
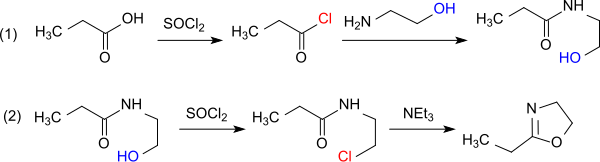
From carboxylic acids , carboxylic acid esters , carboxamides and nitriles , the corresponding N- (2-hydroxyalkyl) carbamides can be obtained with 2-amino alcohols by heating to 200 ° C with dehydration , which on further heating to 260-280 ° C with renewed elimination of water to 2-alkyl -2-oxazoline pass over.
For example, N - (2-hydroxyethyl) propionamide is initially formed from propionic acid and monoethanolamine (MEA) in 74% yield, which can be dehydrated to 2-ethyl-2-oxazoline in approx. 75% yield.
Less drastic reaction conditions require the dehydration of the N- (2-hydroxyethyl) propionamide in vacuo in the presence of iron (III) chloride , which gives the product in 90% yield.
Even higher yields of 96.2% are achieved by heating with zinc acetate dihydrate.
A route that is economical as a one-pot reaction is heating the salt of propionic acid with ethanolamine to 200 ° C. in vacuo in the presence of zinc chloride dihydrate, which gives 2-ethyl-2-oxazoline in a yield of 82%. Pure 2-ethyl-2-oxazoline can be isolated from the water-containing distillate by extraction with diethylbenzene and subsequent distillation or purified by distillation with diethyl phosphite or dimethyldichlorosilane and dried to a residual water content of 10 ppm.
Another one-pot reaction is the three-stage conversion of propionic acid with 2-aminoethanol to form 2-hydroxyethylamide, which reacts with boric acid at 130 ° C to form the boric acid ester, which is thermolysed at 280 ° C in 92% yield to 2-ethyl-2-oxazoline .
The propionyl chloride obtained from propionic acid and thionyl chloride forms with MEA in the presence of z. B. Pyridine as acid scavenger N-propionyl-2-aminoethanol, which reacts with further thionyl chloride to form 2-chloroethylamide. With the chloride ion as the better leaving group, this intermediate is more easily cyclized to the oxazoline by heating. Because of the tendency of oxazolines to open the ring through chloride ions when the imine nitrogen is protonated, work must be carried out with exclusion of water.
The direct reaction of propanoyl chloride with 2-chloroethylamine hydrochloride in the presence of triethylamine avoids the formation of water.
From propionaldehyde
Propionaldehyde reacts with 2-aminoethanol in tert-butanol in the presence of the iodination reagent 1,3-diiodo-5,5-dimethylhydantoin (DIH) and potassium carbonate to form 2-ethyl-2-oxazoline.
properties
2-Ethyl-2-oxazoline is a readily water-soluble, amine-like smelling, colorless liquid that is also soluble in a large number of organic solvents. Aqueous solutions have an alkaline reaction. The compound is stable in alkaline conditions and hydrolyzes under the action of acids.
Applications
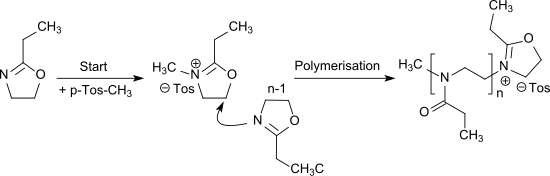
In its anhydrous form, 2-ethyl-2-oxazoline is mainly used as a monomer.
The cationic ring-opening polymerization of 2-ethyl-2-oxazoline is effected by alkylation with e.g. B. methyl tosylate or triflates (especially methyl triflate ) initiates and leads to the water-soluble poly (2-ethyl-2-oxazoline) , which is an N-propionyl-substituted linear polyethyleneimine and can also be regarded as a pseudo-polypeptide.
The polymerization of EOx can also be carried out as living cationic polymerization.
Copolymers with other 2-alkyl-2-oxazolines and other monomers allow the preparation of random copolymers and block copolymers.
The copolymers obtained may be used as biocompatible (engl. Excipient drug carrier ), are used in coatings and adhesives as well as in many other applications.
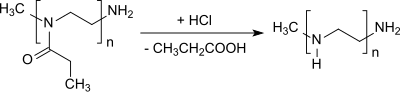
Cleavage of the propionyl group from poly (2-ethyl-2-oxazoline) yields linear polyethyleneimine .
Individual evidence
- ↑ Entry on 2-Ethyl-2-oxazoline at TCI Europe, accessed on June 5, 2016.
- ↑ a b c d e f data sheet 2-ethyl-2-oxazoline from Sigma-Aldrich , accessed on June 5, 2016 ( PDF ).
- ↑ a b c H. Wenker: The synthesis of Δ 2 -oxazolines and Δ 2 -thiazolines from N-acyl-2-aminoethanols . In: J. Amer. Chem. Soc. tape 57 , no. 6 , 1935, pp. 1079-1080 , doi : 10.1021 / ja01309a034 .
- ↑ a b H. Witte, W. Seeliger: Cyclic imidic acid esters from nitriles and amino alcohols . In: Justus Liebigs Ann. Chem. Band 6 , 1974, p. 996-1009 , doi : 10.1002 / jlac.197419740615 .
- ↑ a b Data sheet 2-Ethyl-2-oxazoline from AlfaAesar, accessed on June 5, 2016 ( PDF )(JavaScript required) .
- ↑ a b Etox, 2-Ethyl-2-Oxazoline, Product Information Sheet. Polymer Chemistry Innovations, Inc., accessed July 19, 2016 .
- ↑ a b B.L. Rivas, SI Ananias: Ring-opening polymerization of 2-ethyl-2-oxazoline . In: Polym. Bull. Band 18 , no. 3 , 1987, pp. 189-194 , doi : 10.1007 / BF00255109 .
- ↑ a b c 2-Ethyl-2-oxazoline, Safety Data Sheet. (PDF; 266 kB) (No longer available online.) In: alzchem.com. AlzChem AG, archived from the original on July 21, 2016 ; accessed on June 5, 2016 . Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ a b T. Kagiya, S. Narisawa, T. Maeda, K. Fukui: Ring-opening polymerization of 2-substituted 2-oxazolines . In: J. Polym. Chem., Polym. Lett. tape 4 , no. 7 , 2003, p. 441–445 , doi : 10.1002 / pol . 1966.110040701 .
- ^ R. Hoogenboom: Poly (2-oxazoline) s: A polymer class with numerous potential applications . In: Angew. Chem. Int. Ed. tape 48 , no. 43 , 2009, p. 7978-7994 , doi : 10.1002 / anie.200901607 .
- ↑ Patent US4203900 : Process for preparing 2-oxazolines. Applied on January 3, 1977 , published May 20, 1980 , applicant: The Dow Chemical Co., inventor: ME Kaiser.
- ↑ a b c Patent US4354029 : Preparation of 2-substituted 2-oxazolines with organic zinc salt catalysts. Filed November 30, 1981 , published October 12, 1982 , Applicant: The Dow Chemical Co., Inventor: ME Kaiser, DL Larson.
- ↑ Patent US4281137 : Purification of 2-oxazolines. Applied February 20, 1980 , published July 28, 1981 , Applicants: The Dow Chemical Co., Inventors: JW Sanner, PW Owen.
- ↑ B. Ilkgul, D. Gunes, O. Sirkecioglu, N. Bicak: Synthesis of 2-oxazolines via boron esters of N- (2-hydroxyethyl) amides . In: Tetrahedron Lett. tape 51 , no. 40 , 2010, p. 5313-5315 , doi : 10.1016 / tetlet.2010.07.167 .
- ↑ Holerca MN, Percec V.: 1 H NMR Spectroscopic Investigation of the Mechanism of 2-Substituted-2-Oxazoline Ring Formation and of the Hydrolysis of the Corresponding Oxazolinium Salts . In: Eur. J. Org. Chem. Volume 2000 , no. 12 , 2000, pp. 2257-2263 , doi : 10.1002 / 1099-0690 (200006) 2000: 12 <2257 :: AID-EJOC2257> 3.0.CO; 2-2 .
- ↑ S. Takahashi, H. Togo: An Efficient Oxidative Conversion of Aldehydes into 2-Substituted 2-Oxazolines Using 1,3-Diiodo-5,5-dimethylhydantoin . In: Synthesis . tape 14 , 2009, p. 2329-2332 , doi : 10.1055 / s-0029-1216843 .
- ↑ H. Schlaad, R. Hoogenboom: Poly (2-oxazolines) s and Related Pseudo-Polypeptides . In: Macromol. Chem. Rapid Commun. tape 33 , no. 19 , 2012, p. 1599 , doi : 10.1002 / marc.201200571 .
- ↑ C. Guerrero-Sanchez, R. Hoogenboom, US Schubert: Fast and “green” living cationic ring opening polymerization of 2-ethyl-2-oxazoline in ionic liquids under microwave irradiation . In: Chem. Commun. tape 36 , 2006, p. 3797-3799 , doi : 10.1039 / B608364A .
- ↑ M. Glassner, K. Lava, VR de la Rosa, R. Hoogenboom: Tuning the LCST of poly (2-cyclopropyl-2-oxazoline) via gradient copolymerization with 2-ethyl-2-oxazoline . In: Polym. Chem. Band 52 , no. 21 , 2014, p. 3118-3122 , doi : 10.1002 / pola.27364 .
- ↑ S. Motokucho, M. Furukawa, M. Kawashima, K. Kojio, K. Yoshinaga: Physical properties of poly (tetrahydrofuran) -block-poly (2-ethyl-2-oxazoline) triblock copolymer . In: Polym. J. Band 45 , 2013, p. 1115–1119 , doi : 10.1038 / pj.2013.39 .
- ↑ VR de la Rosa: Poly (2-oxazolines) s as materials for biomedical applications . In: J. Mater. Sci. Mater. Med. Band 25 , no. 5 , 2013, p. 1-15 , doi : 10.1007 / s10856-013-5034-y .
- ↑ Patent US20100197888A1 : Method for manufacturing linear polyethyleneimine (PEI) for transfection purpose and linear PEI obtained with such method. Applied on July 31, 2008 , published on August 5, 2010 , Applicant: Polyplus Transfection, Inventors: A. Adib, F. Stock, P. Erbacher.
- ↑ HML Lambermont-Thijs, FS van der Woerdt, A. Baumgaertel, L. Bonami, FE Du Prez, US Schubert, R. Hoogenboom: Linear Poly (ethylene imine) s by Acidic Hydrolysis of Poly (2-oxazoline) s: Kinetic Screening, Thermal Properties, and Temperature-Induced Solubility Transitions . In: Macromolecules . tape 43 , no. 2 , 2010, p. 927-933 , doi : 10.1021 / ma9020455 .