2-methylene glutaronitrile
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
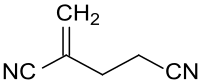
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 2-methylene glutaronitrile | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 6 N 2 | |||||||||||||||
| Brief description |
clear, colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 106.13 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.976 g cm −3 (25 ° C ) |
|||||||||||||||
| Melting point |
−9.6 ° C |
|||||||||||||||
| boiling point |
|
|||||||||||||||
| solubility |
water soluble, soluble in chloroform |
|||||||||||||||
| Refractive index |
1.456 at 25 ° C (589 nm) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
2-Methylenglutaronitril is a dimerization product of acrylonitrile and starting material for di- and triamines , for the biocide 2-bromo-2- (bromomethyl) pentanedinitrile and for heterocycles such. B. 3-cyanopyridine .
Occurrence and representation
In addition to the electrochemical hydrodimerization, the catalytic dimerization of acrylonitrile is also an industrially important reaction for the production of adiponitrile , which after hydrogenation to 1,6-diaminohexane is a key component for technical polymers , such as. B. polyamides (PA 66) or polyurethanes .
If instead of a tail-to-tail link between two acrylonitrile molecules, as in the formation of adiponitrile
a head-to-tail linkage occurs, one obtains in the catalytic dimerization z. B. in the presence of tricyclohexylphosphine PCy 3 in up to 77% yield 2-methyleneglutaronitrile.
As catalysts for the dimerization, metal halides, such as. B. zinc chloride or aluminum chloride with tertiary amines, such as. B. Triethylamine used and achieved crude yields of up to 84%.
When working up, e.g. B. by extraction , and the work-up by distillation are often lost considerable amounts of pure product because of the tendency of 2-methyleneglutaronitrile to polymerize.
In addition to the linear dimerization products 1,4-dicyano-2-butene and 1,4-dicyano-3-butene, which are present as cis-trans isomer mixtures , other oligomers (and polymers) of acrylonitrile usually also occur . The electrochemical hydrooligomerization of acrylonitrile trimers , such as. B. 1,3,6- and 1,3,5-tricyanohexane or tetramers such as 1,3,6,8- and 1,3,5,8-tetracyano-octane.
The reaction of acrylonitrile with tributylphosphine gives 2-methyleneglutaronitrile after fractional distillation in modest yields of approx. 10%. Also, the DABCO catalyzed Acrylnitrildimerisierung with 40% yield of 2,4-dicyano-1-butene after 10 days at room temperature is unproductive.
use
In the older patent literature, processes for isomerizing 2-methyleneglutaronitrile to 1,4-dicyanobutenes as adiponitrile precursors are described, which have become obsolete with the optimization of the electrochemical hydrodimerization of acrylonitrile to adiponitrile.
The electrochemical hydrodimerization of 2-methyleneglutaronitrile produces 1,3,6,8-tetracyano-octane.
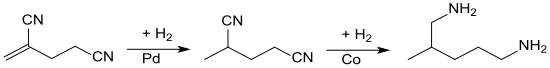
In the hydrogenation of 2,4-dicyano-1-butene, the hydrogen is first added to the double bond in the presence of palladium on activated carbon and 2-methylglutaronitrile is obtained in a practically quantitative yield .
The hydrogenation of the nitrile groups requires more drastic conditions and the presence of ammonia or amines in order to suppress the formation of secondary amines. This second hydrogenation stage is carried out with Raney cobalt as the hydrogenation catalyst and gives 2-methyl-1,5-diaminopentane in 80% yield.
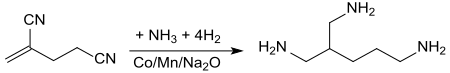
The hydrogenation in the presence of ammonia with manganese-containing and sodium oxide- doped cobalt as a catalyst at 80 to 100 ° C and pressures of 200 atm in a tube reactor converts 2,4-dicyano-1-butene with the addition of ammonia to the double bond directly into 2- Aminomethyl-1,5-pentanediamine with yields of 66%.
The branched triamine can be used in epoxides and polyurethanes.
2-methyleneglutaronitrile reacts with formamide under catalysis with 4- (dimethylamino) pyridine (DMAP) at 60 ° C in 47% yield to 1- ( N -formylamino) -2,4-dicyanobutane, from which α- Aminomethylglutaric acid is formed.
When 2-methyleneglutaronitrile is heated with an alkaline ion exchanger , pyridine and water to 150 ° C. in an autoclave, the lactam 5-cyano-2-piperidone is produced in 80% yield .
Homopolymers and copolymers can also be produced with 2-methyleneglutaronitrile by anionic polymerization with sodium cyanide , sodium in liquid ammonia or with butyllithium , which, however, are only obtained in very low yields and have unsatisfactory properties, such as e.g. B. have low inherent viscosities and poor mechanical properties.
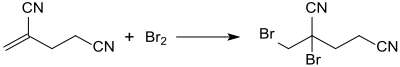
The most important use of 2-methyleneglutaronitrile is as a starting material for the broad-spectrum biocide 2-bromo-2- (bromomethyl) pentanine nitrile (methyldibromoglutaronitrile), which is formed in practically quantitative yield when bromine is added to the double bond.
From the chlorine-analogous 2-chloro-2- (chloromethyl) pentanedinitrile, 3-cyanopyridine is obtained by heating to 150 ° C. with tin (IV) chloride .
Individual evidence
- ↑ a b c Entry on 2-methyleneglutaronitrile at TCI Europe, accessed on June 25, 2017.
- ↑ a b c d e data sheet 2-methylenglutaronitrile from Sigma-Aldrich , accessed on June 25, 2017 ( PDF ).
- ↑ a b L. Yu et al .: Practical and scalable preparation of 2-methyleneglutaronitrile via an efficient and highly selective head-to-tail dimerization of acrylonitrile catalysed by low-loading of tricyclohexylphosphine . In: RSC Adv. Band 4 , 2014, p. 19122-19126 , doi : 10.1039 / C4RA02810D .
- ^ William M. Haynes: CRC Handbook of Chemistry and Physics, 97th Edition . CRC Press, Boca Raton, FL, USA 2017, ISBN 978-1-4987-5429-3 , pp. 3-370 .
- ^ Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons, 2nd Edition . Elsevier Inc., Oxford, UK 2015, ISBN 978-0-323-28659-6 , pp. 100 .
- ↑ a b Patent US3733351 : Production of 2-methylene-glutaronitrile. Applied on April 25, 1969 , published May 15, 1973 Applicant: Mitsubishi Petrochemical Co., Inventors: Y. Watanabe, M. Takeda.
- ↑ Patent US4422981 : Process for production of 2-methyleneglutaronitrile. Applied March 24, 1982 , published December 27, 1983 , Applicant: Mitsubishi Petrochemical Co., Inventor: H. Omori, M. Takeda, K. Fujita, M. Kataoka.
- ↑ Patent US3956358 : Dimerization method. Filed December 30, 1974 , published May 11, 1976 , applicant: Halcon International, Inc., inventor: OT Onsager.
- ↑ MM Baizer, JD Anderson: Electrolytic reductive coupling. VII. A new class of acrylonitrile oligomers . In: J. Org. Chem. Band 30 , no. 5 , 1965, pp. 1351-1356 , doi : 10.1021 / jo01016a003 .
- ↑ a b M.M. Baizer, JD Anderson: Electrolytic reductive coupling. VIII. Utilization and a new preparation of α-methylene-glutaronitrile . In: J. Org. Chem. Band 30 , no. 5 , 1965, pp. 1357-1360 , doi : 10.1021 / jo01016a004 .
- ↑ D. Basavaiah, VVL Gowriswari, TK Barathi: DABCO catalyzed dimerization of α, β- unsaturated ketones and nitriles . In: Tetrahedron Lett. tape 28 , no. 39 , 1987, pp. 4591-4592 , doi : 10.1016 / S0040-4039 (00) 96573-0 .
- ↑ Patent US3795694 : Preparation of cyano compounds. Applied on October 16, 1972 , published March 5, 1974 , applicant: Halcon International, Inc., inventor: OT Onsager.
- ↑ Patent US3350439 : Process for preparing aminoalkanenitriles. Filed June 1, 1965 , published October 31, 1967 , Applicant: National Distillers and Chemical Corp., Inventor: J. Feldman, M. Thomas.
- ↑ Patent US3408397 : Methyl pentamethylene diamine process. Filed June 1, 1965 , published October 31, 1967 , Applicant: National Distillers and Chemical Corp., Inventor: J. Feldman, M. Thomas.
- ↑ Patent EP1028104A1 : Process for the production of 2-aminomethyl-1,5-pentanediamine. Applied on January 27, 2000 , published on August 16, 2000 , applicant: Bayer AG, inventors: K. Fischer, F. Richter, A. Bazanov, A. Timofeev, N. Zubritskaya, G. Smirnova.
- ↑ Patent EP0336185A1 : 1- (N-Formylamino) -2,4-dicyanobutane and a process for its production. Registered on March 18, 1989 , published on October 11, 1989 , applicant: Bayer AG, inventor: H.-J. Scholl.
- ↑ Patent US3666766 : Selective hydrolysis and cyclization of unsaturated nitriles. Applied on December 27, 1967 , published May 30, 1972 , Applicant: National Distillers and Chemical Corp., Inventor: JB Pedigo, J. Feldman, IA Kereszies.
- ↑ Patent US3451977 : Process for polymerizing 2-methylene glutaronitrile. Applied July 27, 1964 , published June 24, 1969 , Applicant: National Distillers and Chemical Corp., Inventor: JM Hoyt, K. Koch.
- ↑ a b Patent US3644380 : Preparation of 3-cyanopyridine. Applied on November 24, 1969 , published February 22, 1972 , Applicant: Merck & Co., Inc., Inventor: R. Harmetz, RJ Tull.
- ↑ Patent US3929858 : Method for preparing 2-bromo-2-bromomethyl-glutaronitrile. Applied on December 3, 1974 , published December 30, 1975 , applicant: Merck & Co., Inc., inventor: RD Swigert.