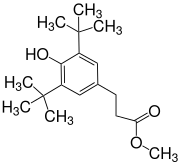
3- (3,5-Di- tert- butyl-4-hydroxyphenyl) propionic acid methyl ester
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 3- (3,5-Di- tert- butyl-4-hydroxyphenyl) propionic acid methyl ester | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 18 H 28 O 3 | |||||||||||||||
| Brief description |
white crystal powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 292.42 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.09 g cm −3 |
|||||||||||||||
| Melting point |
66.1 ° C |
|||||||||||||||
| boiling point | ||||||||||||||||
| Vapor pressure |
0.00002 hPa at 25 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
3- (3,5-Di- tert- butyl-4-hydroxyphenyl) propionic acid methyl ester ( Metilox ) is a derivative of 4-hydroxycinnamic acid , which is hydrogenated to 3- (4-hydroxyphenyl) propanoic acid (4-hydroxydihydrocinnamic acid) and esterified to the methyl ester . As a sterically hindered phenol , Metilox has antioxidant properties and is the starting compound for longer-chain and more complex esters and amides. a. be used as stabilizers for polymers.
Manufacturing
In the synthesis of methyl- di - tert- butylhydroxyhydrocinnamate ( 2 ), potassium tert -butanolate is used to generate the mesomeric anion from 2,6-di- tert- butylphenol ( 1 ) , which in its carbanion form in a Michael addition reacts with methyl acrylate . After acidification, the product can be isolated as a yellowish oil by vacuum distillation , which solidifies to a white solid.
properties
3- (3,5-Di- tert- butyl-4-hydroxyphenyl) propionic acid methyl ester is a white solid which is initially obtained as a yellowish oil during synthesis. The compound is practically insoluble in water, but dissolves in many organic solvents. Its poor biodegradability is problematic if it should get into the environment as a component of liquid mixtures.
Applications
Like its starting compound 2,6-di- tert- butylphenol, methyl 3- (3,5-di- tert- butyl-4-hydroxyphenyl) propionate is also effective as a radical scavenger as an antioxidant in polymers, especially in polyolefins and polyurethanes . It is also used in technical oils, such as. B. engine oils , lubricating oils and gear oils , used in hydraulic fluids and cooling lubricants ( English metal working fluids , MWF). Metilox is an intermediate for a number of hindered phenols with longer alkyl chains or spatially more bulky structures in order to reduce volatility and diffusion in polymers and to suppress fogging , especially in vehicle interiors and living spaces.
The higher esters of Metilox (Octyl = Irganox 1135, Octadecyl = Irganox 1076, Pentaerythrityl = Irganox 1010) are mostly obtained by transesterification with the respective alcohol under catalysis with z. B. alkali hydroxides , such as lithium hydroxide or with organic tin compounds , such as dibutyltin oxide .
The Michael addition of higher acrylic acid esters, such as. B. Octadecyl acrylate on 2,6-di- tert- butylphenol analogous to the Metilox synthesis is also mentioned, but not technically relevant.
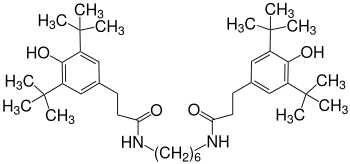
Amides (instead of esters) such as the diamide of 1,6-diaminohexane (Irganox 1098) are particularly suitable for stabilizing polyamides, such as. B. polyamide 6 .
The most efficient possible stabilization of polymers against oxidative degradation initiated by light and heat usually requires the addition of anti- aging agents as mixtures (so-called " stabilizer packages ") of hindered phenols and other compounds, such as the antioxidant phosphonates , radical scavengers such as HALS ( hindered amine light stabilizers ) and UV absorbers such as benzophenones , benzotriazoles or hydroxyphenyl triazines .
literature
- George Wypych (Ed.): Handbook of UV Degradation and Stabilization, 2nd Edition . ChemTec Publishing, Toronto 2015, ISBN 978-1-895198-86-7 .
Individual evidence
- ↑ a b c d e f g h OECD : Screening Information Dataset (SIDS) Initial Assessment Report (SIAR) for Metilox , accessed on June 15, 2018.
- ↑ a b c Patent US3364250 : Methyl β- (3,5-di-tert-butyl-4-hydroxyphenyl) propionate. Applied October 22, 1965 , published January 16, 1968 , Applicant: Geigy Corp., Inventor: M. Dexter, EA Meier.
- ↑ Entry on Methyl 3- (3,5-di-tert-butyl-4-hydroxyphenyl) propionate at Toronto Research Chemicals , accessed October 1, 2018 ( PDF ).
- ↑ a b fluorochem: MSDS Methyl 3- (3,5-di-tert-butyl-4-hydroxyphenyl) -propionate , accessed June 25, 2018.
- ↑ Patent US7985871B2 : Preparation of tetrakis [3 (3,5-di-tert-butyl-4-hydroxyphenyl) propionyloxymethyl] methane. Filed August 6, 2009 , published July 26, 2011 , applicant: Chemtura Corp., inventor: JS Hill.
- ↑ Patent US4594444 : Process for the preparation of sterically hindered hydroxyphenylcarboxylic acid esters. Applied on December 12, 1984 , published June 10, 1986 , applicant: Ciba-Geigy Corp., inventor: I. Orban.
- ↑ Patent EP0808818A1 : Process for the preparation of substituted hydroxy-hydrocinnamate esters. Applied on May 16, 1997 , published November 26, 1997 , Applicant: Ciba Specialty Chemicals Holding Inc., Inventors: ME Schultz, B. Dubuis, P. Küng, JR Ross.
- ↑ Patent US3677965 : Alkylhydroxyphenyl polyamides as antioxidants. Applied on December 29, 1967 , published July 18, 1972 , Applicants: Geigy Corp., Inventors: M. Dexter, B. Manor, JD Spivack, DH Steinberg.