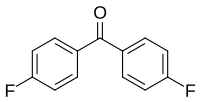
4,4'-difluorobenzophenone
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 4,4'-difluorobenzophenone | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 13 H 8 F 2 O | |||||||||||||||
| Brief description |
white to light yellow crystalline solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 218.20 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density | ||||||||||||||||
| Melting point | ||||||||||||||||
| boiling point | ||||||||||||||||
| solubility |
very slightly soluble in water (0.00876 g · l −1 at 20 ° C ), soluble in ethanol and toluene |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
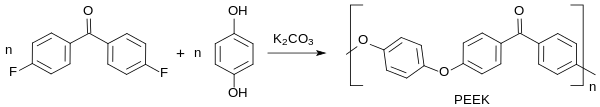
4,4'-Difluorobenzophenone is a benzophenone derivative in which both hydrogen atoms opposite the ketone group (in the para position ) have been replaced by fluorine atoms . The compound is the starting material for polyether ketones , PEK and polyether ether ketones PEEK, which are characterized by high glass transition and very high melting temperatures (T m > 330 ° C).
Occurrence and representation
The synthesis of 4,4'-difluorobenzophenone was first reported in 1933. In the Friedel-Crafts acylation of fluorobenzene with the acid chloride of 4-fluorobenzoic acid in the presence of the acylation catalyst aluminum chloride AlCl 3 , 4,4'-difluorobenzophenone and small amounts of the isomeric 2,4'-difluorobenzophenone are produced in 52% yield .
When using a mixture of aluminum chloride and lithium chloride in 1,2-dichloroethane , 4,4'-DFBP is obtained in 94.5% yield and 97.2% purity.
The necessary use of superstoichiometric amounts of the acylation catalyst AlCl 3 and the costs of disposing of its hydrolysis products stimulated the search for alternative synthetic routes early on.
Diazotization of 4,4'-diaminodiphenylmethane in 95% aqueous hydrofluoric acid gives 4,4'-difluorodiphenylmethane as an intermediate product, which can be oxidized to 4,4'-difluorobenzophenone in a pure yield of 78% by heating with excess sodium nitrite for several hours .
The use of highly concentrated hydrofluoric acid is problematic from a process and safety point of view.
During the thermal decomposition ( Balz-Schiemann reaction ) of the tetrafluoroborate salt of the diazotized 4,4'-diaminodiphenylmethane isolated as a solid, 4,4'-difluorodiphenylmethane is formed with the elimination of nitrogen and boron trifluoride , the methylene group of which is in turn oxidized to the ketone 4,4'-DFBP .
Another process uses the acid-catalyzed reaction of formaldehyde with fluorobenzene with fluorobenzenesulfonic acid to form a difluorodiphenylmethane mixture, which consists of 77% of 4,4'-DFBP and 23% of the isomeric 2,4'-DFBP. The mixture is oxidized with concentrated nitric acid and purified by recrystallization with a 9: 1 glacial acetic acid / water mixture. Recrystallization three times gives 4,4'-DFBP in 99.5% purity.
In view of the lack of information on the yield of the individual process stages, the high proportion of undesired isomers and their laborious separation, this synthesis route does not seem to be of much use.
Because of the high purity requirements for 4,4'-DFBP as a monomer for polycondensation reactions and the expensive fluoroaromatic compounds used, further alternative routes to 4,4'-difluorobenzophenone have been proposed, but these have not found industrial application. By-products, in particular the undesired isomer 2,4'-DFBP and the nitroaromatics formed when sodium nitrite and nitric acid are used, must be separated off as quantitatively as possible before using 4,4'-DFBP as a monomer.
properties
4,4'-Difluorobenzophenone is an odorless white crystal powder that dissolves in ethanol and toluene. Impurities such as B. the most common by-product 2,4'-difluorobenzophenone can be removed by (multiple) recrystallization from cyclohexane or from an 80:20 ethanol: water mixture.
Applications
4,4'-Difluorobenzophenone is a key raw material for the polyetheretherketone PEEK, by far the most important thermoplastic high-performance plastic from the class of polyaryletherketones PAEK.
The polycondensation between 4,4'-difluorobenzophenone and the alkali metal carbonates, z. B. potassium carbonate formed alkali metal salt of hydroquinone to PEEK takes place in diphenyl sulfone as a solvent by stepwise heating under protective gas up to 320 ° C.
An almost colorless polymer with T g of approx. 140 ° C and T m of 334 ° C is formed.
PEEK is increasingly being used as a metal replacement material due to its extraordinary thermal stability from −196 to +260 ° C, its high solvent and chemical resistance, and its good biocompatibility.
Individual evidence
- ↑ a b c d e f g h i j data sheet 4,4′-Difluorobenzophenone from Sigma-Aldrich , accessed on May 2, 2019 ( PDF ).
- ↑ a b Entry on 4,4'-Difluorobenzophenone at TCI Europe, accessed on May 2, 2019.
- ↑ Data sheet 4,4'-Difluorobenzophenone, 98% from AlfaAesar, accessed on May 2, 2019 ( PDF )(JavaScript required) .
- ^ RD Dunlop, JH Gardner: The preparation of 4-fluoro- and 4,4'-difluorobenzophenone . In: J. Am. Chem. Soc. tape 55 , no. 4 , 1933, pp. 1665–1666 , doi : 10.1021 / ja01331a058 .
- ↑ Patent US4814508 : Friedel-Crafts preparation of aromatic ketones. Applied March 21, 1988 , published March 21, 1989 , Applicant: Raychem Corp., Inventor: HC Gors, PJ Horner, J. Jansons.
- ↑ a b Patent EP0004710B1 : Preparation of 4,4'-difluorobenzophenone. Applied March 8, 1979 , published November 4, 1981 , Applicant: Imperial Chemical Industries PLC, Inventor: PA Staniland, RD Bowden, L. Burgess, RJ Clark.
- ↑ RE Banks, ed .: Fluorine Chemistry at the Millenium - Fascinated by Fluorine . Elsevier, Amsterdam 2000, ISBN 0-08-043405-3 , pp. 177 .
- ↑ Patent US7687668B2 : Process for preparing 4,4'-difluorobenzophenone. Registered on July 27, 2006 , published on March 30, 2010 , applicant: Evonik Fibers GmbH, inventors: H. Rögl, M. Ungerank.
- ↑ Patent US5777172 : Process for the preparation of benzophenthiones and benzophenones. Applied on May 16, 1995 , published on July 7, 1998 , applicant: Zeneca Ltd., inventor: MCH Standen, NC Evens.
- ↑ Patent EP0001879A1 : Thermoplastic aromatic polyetherketones, a method for their preparation and their application as electrical insulants. Applied on June 22, 1978 , published May 16, 1979 , Applicant: Imperial Chemical Industries Ltd., Inventor: JB Rose, PA Staniland.
- ↑ Polyaryletherketones (PAEK). In: SPECIAL K 2016 world of plastics. Carl Hanser Verlag, October 2016, accessed on May 5, 2018 .