Bis (trimethylsilyl) carbodiimide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Bis (trimethylsilyl) carbodiimide | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 7 H 18 N 2 Si 2 | |||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 186.40 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.8211 g cm −3 at 25 ° C |
|||||||||||||||
| Melting point |
−24 ° C to −23 ° C |
|||||||||||||||
| boiling point |
164 ° C |
|||||||||||||||
| solubility |
miscible with diethyl ether , 1,4-dioxane , gasoline, benzene and tetrachloromethane |
|||||||||||||||
| Refractive index |
1.4251 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Bis (trimethylsilyl) carbodiimide is a carbodimide in which both hydrogen atoms of the unstable parent compound carbodiimide HN = C = NH have been replaced by trimethylsilyl groups. BTSC is a highly reactive equivalent for cyanamide and is suitable for the synthesis of active ingredients and dyes that contain a cyanimino group = N-CN, for the simple preparation of amidines and technical ceramics based on silicon nitrides .
Manufacturing
The synthesis of bis (trimethylsilyl) carbodiimide was first reported in 1962 by Ulrich Wannagat's group . Several synthesis variants were processed, such as B.
- Bis-trimethylsilyl urea Me 3 SiNHCONHSiMe 3 + phenyllithium C 6 H 5 Li + trimethylchlorosilane Me 3 SiCl (yield> 80%)
- Bis-trimethylsilylurea + sodium amide NaNH 2 + trimethylchlorosilane (yield 12%)
- Sodium bis trimethylsilyl amide (Me 3 Si) 2 NNa + phosgene COCl 2 (yield 60%)
- Sodium bis trimethylsilyl amide + silicon tetraisocyanate Si (NCO) 4 (yield 20%)
- Disilbercyanamid Ag 2 CN 2 + trimethylchlorosilane (yield 90%)
some of which give good yields, but require expensive or uncomfortable to handle reagents.
Another variant - the desulfurization of bis-trimethylsilyl-thiourea with silver - imidazole - does not represent any improvement with a 51% yield, while bis (trimethylsilyl) carbodiimide was obtained in 81% yield from cyanamide and trichloromethylsilane in the presence of triethylamine .
With trimethylsilyl cyanide , cyanamide reacts within one minute to BTSC in 90% yield.
A technical process converts hexamethyldisilazane with cyanamide at room temperature to BTSC in 90% yield and high purity (99.5%) and seems to be the most promising synthesis variant.
properties
Bis (trimethylsilyl) carbodiimide is a flammable, clear and colorless liquid which is stable at room temperature and which is decomposed by water, splitting the Si-N bond and forming hexamethyldisiloxane and cyanamide. In contrast to organic diimides, BTSC does not react with hydrogen sulfide H 2 S, ammonia NH 3 or aniline . The compound is miscible with many organic solvents .
Applications
In contrast to organic diimines, such as in particular dicyclohexylcarbodiimide, bis (trimethylsilyl) carbodiimide can not be used for the synthesis of amides or esters by splitting off water.
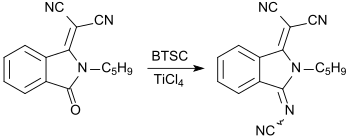
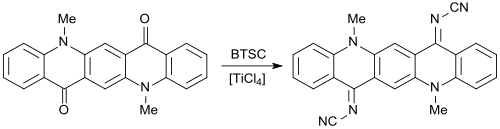
With bis (trimethylsilyl) carbodiimide, carbonyl groups C = O can be converted into cyanimino groups N-CN. As reported for the first time in 1984 by Siegfried Hünig's working group, (substituted) 1,4-benzoquinones give rise to strong electron acceptors , which, in analogy with tetracyanoquinodimethane TCNQ, with electron donors , such as e.g. B. Form tetrathiafulvalene TTF charge transfer complexes that have superconducting properties .
The electrochemical behavior of the cyanimino group is largely identical to that of the spatially larger dicyanomethylene group. It can avoid bulky substituents and better maintain the planar structure that is important for the formation of CT complexes.
The standard compound TCNQ can be obtained by means of TMSCN from terephthalic acid dichloride in the presence of pyridine via the silylated benzene derivative and subsequent reaction with phosphorus oxychloride in a total yield of approx. 60%.
Hybrid electron acceptors with dicyanomethylene and cyanimino groups have also been described, which, as yellow solids, form green CT complexes with the orange TTF.
When replacing ketone functions with cyanimino groups in pigments , such as. B. quinacridone pigments , fluorescent dyes are obtained.
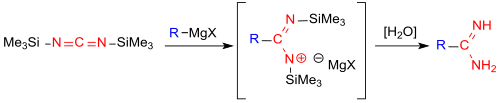
The direct introduction of a carboxamidine function -CO (NH) NH 2 into aliphatic and aromatic Grignard compounds has recently been reported.
The reaction with bis (trimethylsilyl) carbodiimide avoids the multistage preparation of the imino ester salts from nitriles and alcohols (Pinner salts) and their reaction with ammonia in the Pinner reaction . However, the amidine salts which can be obtained in high yields using the new process are contaminated with magnesium halides, which can only be separated off with high yield losses .
By chemical vapor deposition , bis (trimethylsilyl) carbodiimide can be used to produce ceramic Si-CN films on steel surfaces - e.g. B. for hardening cutting tools - which turned out to be extremely corrosion-resistant, but also brittle and poorly adhering.
literature
Henri Ulrich: Chemistry and Technology of Carbodiimides . Wiley-VCH, Weinheim 2007, ISBN 978-0-470-06510-5 .
Individual evidence
- ↑ a b c d data sheet bis (trimethylsilyl) carbodiimide from Sigma-Aldrich , accessed on July 3, 2018 ( PDF ).
- ↑ a b c d e f g J. Pump, U. Wannagat: Contributions to the chemistry of silicon-nitrogen compounds, XIV, bis-trimethylsilyl-carbodiimide . In: Justus Liebigs Ann. Chem. Band 652 , no. 1 , 1962, pp. 21-27 , doi : 10.1002 / jlac.19626520104 .
- ↑ J. Pump, U. Wannagat: Bis- (trimethylsilyl) -carbodiimide . In: Angew. Chem. Band 74 , no. 3 , 1962, pp. 117-117 , doi : 10.1002 / anie.19620740308 .
- ↑ L. Birkofer, A. Ritter, P. Richter: N, N'-Bis-trimethylsilyl-carbodiimide . In: Tetrahedron Lett. tape 3 , no. 5 , 1962, pp. 195-198 , doi : 10.1016 / S0040-4039 (00) 70856-2 .
- ↑ K. Mai, G. Patil: An expedient synthesis of bis (trimethylsilyl) carbodiimide . In: J. Org. Chem. Band 52 , no. 2 , 1987, pp. 275-276 , doi : 10.1021 / jo00378a020 .
- ↑ Patent DE19822281C1 : Process for the production of bis-trimethylsilyl-carbodiimide. Registered on May 18, 1998 , published on August 19, 1999 , applicant: SKW Trostberg AG, inventor: T. Güthner, H. Krommer.
- ↑ A. Aumüller, S. Hünig: One-step route to N ‐ cyanimines and to N, N′ ‐ dicyanchinonediimines, a new class of electron acceptors . In: Angew. Chem. Band 96 , no. 6 , 1984, pp. 437-438 , doi : 10.1002 / anie.19840960620 .
- ↑ A. Aumüller, S. Hünig: Multistep Reversible Redox Systems, XLVI 1 ) N, N′ ‐ Dicyanoquinonediimines - A New Class of Compounds, I: Synthesis and General Properties . In: Justus Liebigs Ann. Chem. Band 142 , 1986, pp. 142-164 , doi : 10.1002 / jlac.198619860114 .
- ↑ S. Yamaguchi, T. Hanafusa: Preparation of 7,7,8,8-tetracyanoquinodimethane and its derivatives . In: Chem. Lett. tape 14 , no. 6 , 1985, pp. 689-690 , doi : 10.1246 / cl.1985.689 .
- ↑ Synthesis of heterocyclic analogues of Benzo-TCQN. In: Ph.D. Thesis. Dublin City University, July 2002, accessed July 16, 2018 .
- ↑ Patent EP0673979B1 : fluorescent dyes containing cyanoimino groups. Applied March 16, 1999 , published August 30, 2000 , Applicant: Ciba Specialty Chemicals Holding Inc., Inventors: JS Zambounis, Z. Hao, A. Iqbal.
- ↑ T. Güthner, E. Huber, J. Sans, F. Thalhammer: Direct introduction of an N, N'-nonsubstituted carboxamidine group by Grignard addition to silylated cyanamide . In: Synlett . tape 28 , no. 12 , 2017, p. 1437-1440 , doi : 10.1055 / s-0036-1589000 .
- ↑ Y. Zhou et al .: Hard silicon carbonitride films obtained by RF-plasma-enhanced chemical vapor deposition using the single-source precursor bis (trimethylsilyl) carbodiimide . In: J. Eur. Ceram. Soc. tape 26 , 2006, p. 1325-1335 , doi : 10.1016 / j.jeurceramsoc.2005.02.004 .