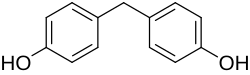
Bisphenol F
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Bisphenol F | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 13 H 12 O 2 | ||||||||||||||||||
| Brief description |
reddish crystalline solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 200.20 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
162-164 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Bisphenol F ( BPF for short ) is an aromatic organic compound from the group of diphenylmethane derivatives and one of the bisphenols . In BPF, the two aromatic rings are linked by a methylene group (CH 2 ).
use
BPF is used to make epoxy resins and coatings, which are used in industry to increase the thickness and durability of materials, such as in:
- Tank and pipe linings
- Industrial floors
- Road surfaces
- Structural adhesives
- Grout .
BPF epoxy resins are also used in various consumer products:
In the dental sector, BPF epoxy resins are used in or for dental materials: as restorative material, for linings, as an adhesive , for the production of prostheses and tissue substitutes.
Metabolism and Toxicity
Studies have shown that BPF is primarily glucoronidated and sulfated in phase II biotransformation reactions , the type of metabolism depending on the cell type. In cell models, the corresponding sulfates were mainly formed in a human hepatoma cell line (HepG2), whereas both sulfates and glucuronides were formed in hepatocytes . In addition, various hydroxylated BPF metabolites are formed in phase I biotransformation reactions , including mainly meta- hydroxylated BPF, ortho- hydroxylated BPF and 2,4-dihydroxybenzophenone (DHB). These metabolic pathways are P450 dependent.
In a rat study, 43–54% of the dose was excreted in the urine and 15–20% in the faeces of BPF and its metabolites within 96 hours ; the remainder of the administered dose remained in various organs of the rat and was recovered mainly in the lumen of the digestive tract and in the liver . In pregnant rats, BPF has also been found in the uterus , placenta , amniotic fluid, and fetuses , indicating that BPF can cross the placental barrier. Another rat study showed liver toxicity as the main effect .
A review of the hormonal activity of the bisphenols A, S and F found in four out of five in vivo - studies reports of estrogenic , androgenic and thyroidogene effects for BPF. In vitro - studies on BPF showed effects such as cytotoxicity , cellular dysfunction , DNA damage and chromosome aberration .
Occurrence and release
BPF has been shown to be present in the environment and in food contact material.
But the main source of intake in the general population is likely mustard . BPF is created in the production of mild mustard from natural ingredients. In this case, BPF is a natural ingredient and not a contamination from food contact material. This leads to a low chronic exposure of humans. Because of this chronic exposure and the proven estrogenic effects of BPA, studies on BPF have been and are being carried out to assess the effects of BPF on living organisms . The cytotoxicity and genotoxicity of BPF and some of its metabolites have been characterized, with BPF exhibiting intermediate cytotoxicity. BPF was not found to produce a genetic mutation when tested via an Ames test. However, when human cell lines were tested and a comet assay was performed, BPF caused DNA fragmentation when introduced into cells at non-cytotoxic concentrations. In addition, another study found that BPF is genotoxic when introduced into Hep-G2 cells.
Individual evidence
- ↑ a b c d e data sheet bis (4-hydroxyphenyl) methane from Sigma-Aldrich , accessed on April 11, 2020 ( PDF ).
- ↑ a b c d e f Johanna Ruth Rochester, Ashley Louise Bolden: Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes . In: Environmental Health Perspectives . 123, No. 7, 2015, pp. 643-50. doi : 10.1289 / ehp.1408989 . PMID 25775505 . PMC 4492270 (free full text).
- ↑ a b c Nicolas Cabaton, Marie-Christine Chagnon, Jean-Claude Lhuguenot, Jean-Pierre Cravedi, Daniel Zalko: Disposition and Metabolic profiling of bisphenol F in pregnant and nonpregnant rats . In: Journal of Agricultural and Food Chemistry . 54, No. 26, December 27, 2006, pp. 10307-10314. doi : 10.1021 / jf062250q . PMID 17177575 .
- ↑ a b c Nicolas Cabaton, Daniel Zalko, Estelle Rathahao, Cécile Canlet, Georges Delous, Marie-Christine Chagnon, Jean-Pierre Cravedi, Elisabeth Perdu: Biotransformation of bisphenol F by human and rat liver subcellular fractions . In: Toxicology in Vitro . 22, No. 7, October 1, 2008, pp. 1697-1704. doi : 10.1016 / j.tiv.2008.07.004 . PMID 18672047 .
- ^ A b Coralie Dumont, Elisabeth Perdu, Georges de Sousa, Laurent Debrauwer, Roger Rahmani, Jean-Pierre Cravedi, Marie-Christine Chagnon: Bis (hydroxyphenyl) methane — bisphenol F — metabolism by the HepG2 human hepatoma cell line and cryopreserved human hepatocytes . In: Drug and Chemical Toxicology . 34, No. 4, October 1, 2011, pp. 445-453. doi : 10.3109 / 01480545.2011.585651 . PMID 21770713 .
- ↑ a b c d Marc Audebert, L. Dolo, E. Perdu, J.-P. Cravedi, D. Zalko: Use of the γH2AX assay for assessing the genotoxicity of bisphenol A and bisphenol F in human cell lines . In: Archives of Toxicology . 85, No. 11, June 9, 2011, pp. 1463-1473. doi : 10.1007 / s00204-011-0721-2 . PMID 21656223 .
- ↑ Nobuhiko Higashihara, Keiji Shiraishi, Katusi Miyata, Yutaka Oshima, Yasushi Minobe, Kanji Yamasaki: Subacute oral toxicity study of bisphenol F based on the draft protocol for the "Enhanced OECD Test Guideline no. 407" . In: Archives of Toxicology . 81, No. 12, December 1, 2007, pp. 825-832. doi : 10.1007 / s00204-007-0223-4 . PMID 17628788 .
- ↑ a b c Nicolas Cabaton, Coralie Dumont, Isabelle Severin, Elisabeth Perdu, Daniel Zalko, Mustapha Cherkaoui-Malki, Marie-Christine Chagnon: Genotoxic and endocrine activities of bis (hydroxyphenyl) methane (bisphenol F) and its derivatives in the HepG2 cell line . In: Toxicology . 255, No. 1-2, January 8, 2009, pp. 15-24. doi : 10.1016 / j.tox.2008.09.024 . PMID 18973785 .
- ^ O. Zoller, BJ Brüschweiler, R. Magnin, H. Reinhard, P. Rhyn, H. Rupp, S. Zeltner, R. Felleisen: Natural occurrence of bisphenol F in mustard . In: Food Additives & Contaminants: Part A . 33, No. 1, November 23, 2015, pp. 137–146. doi : 10.1080 / 19440049.2015.1110623 . PMID 26555822 .
