Palmitoleic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
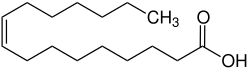
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Palmitoleic acid | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 16 H 30 O 2 | |||||||||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 254.41 g · mol -1 | |||||||||||||||||||||
| Physical state |
liquid |
|||||||||||||||||||||
| density |
0.90 g cm −3 |
|||||||||||||||||||||
| Melting point |
1 ° C |
|||||||||||||||||||||
| boiling point |
210 ° C (at 13 h Pa ) |
|||||||||||||||||||||
| solubility |
poorly soluble in water |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Palmitoleic acid (chemically: cis -hexadec-9-enoic acid ) is a monounsaturated fatty acid with 16 carbon atoms. The double bond is between C 9 and C 10 or, viewed from the end of the carbon chain, on the seventh carbon atom, so it is an omega-7 fatty acid . Monoenoic acid is one of the non-essential fatty acids.
It was first found in 1854 by Gotthard Hofstädter in the Walrat and known under the name of physetoleic acid . The current name was proposed in 1927, almost at the same time it was discovered that zoomaric acid is identical.
Occurrence
Palmitoleic acid is often found in vegetable fats, mostly in smaller amounts up to approx. 10%. It occurs in larger quantities (chemically bound to glycerine ) as glyceride in animal tissues such as depot fat from birds, rodents and some mammals, as well as reptiles and amphibians , in fish and marine animal oils, as well as whale rat and in mammalian liver .
In vegetable fats and oils , triacylglycerides containing palmitoleic acid occur in high concentrations in sea buckthorn oil of up to over 32% of the fatty acid residues, in macadamia oil and in avellana oil of the Chilean hazelnut about 20% of the fatty acid residues. They are therefore used as a substitute for mink oil, which contains around 17-20%. It occurs in large quantities in various algae oils and in the seed oil of the jackalberry Diospyros mespiliformis , in the pulp of the sugar melon ( Cucumis melo ), papaya ( Carica papaya ) and persimmon ( Diospyros kaki ). It is also found in higher concentrations in the fatty acids in baker's yeast .
Palmitoleic acid is also contained in the fraction of free fatty acids of the stratum corneum (the outermost layer of the epidermis ) at just under 4%, which is why it is in demand in cosmetic applications and leather care. It is a lipokine with a hormone-like effect, the name for palmitoleic acid as a lipid released by adipose tissue with a hypothetical hormone effect.
The biosynthesis takes place starting from palmitic acid by desaturation, i.e. the conversion of saturated into unsaturated compounds by means of the enzyme delta-9- desaturase (SCD). It is also produced by β-oxidation from vaccenic acid , the reverse case, the elongase of vaccenic acid produces palmitoleic acid again.
Isomers
A number of isomers of palmitoleic acid are found in vegetable sources. The cis isomers (4 Z ) -, (5 Z) - , (7 Z ) - (hypogaic acid), (8 Z ) -, (10 Z ) -, (11 Z ) -hexadecenoic acid, and the trans (3 E ) -Hexadecenoic acid.
It occurs in natural sources as a trans stereoisomer, i.e. palmitelaidic acid ( E ) -9-hexadecenoic acid, before z. B. in dairy products.
In human sebum , more than 20% of the special regioisomer, sapienic acid (C16: 1-delta-6c) occurs, which is produced here by a delta 6 desaturase of palmitic acid .
Individual evidence
- ↑ a b c d e Entry for CAS no. 373-49-9 in the GESTIS substance database of the IFA , accessed on November 12, 2006(JavaScript required) .
- ↑ a b Datasheet Palmitoleic acid from Sigma-Aldrich , accessed on April 16, 2011 ( PDF ).
- ^ Georg Löffler: Functional Biochemistry. Springer, 1993, ISBN 978-3-540-54692-4 , p. 136.
- ↑ a b H. Schönfeld (Ed.): Chemistry and technology of fats and fat products. 1. Volume, Springer, 1936, ISBN 978-3-7091-5855-5 (reprint), p. 27 f.
- ↑ Walter Karrer: Constitution and occurrence of organic plant substances. 2nd edition, Springer, 1976, ISBN 978-3-0348-5143-5 , p. 303.
- ↑ 9-Hexadecenoic acid from PlantFA Database, accessed November 2, 2017.
- ↑ Josef Schormüller : The components of food. Springer, 1965, ISBN 978-3-642-46012-8 , p. 321.
- ↑ a b Sabine Krist: Lexicon of vegetable fats and oils. 2nd edition, Springer, 2013, ISBN 978-3-7091-1004-1 , pp. 98, 433, 706.
- ^ Michael A. Borowitzka, Navid R. Moheimani: Algae for Biofuels and Energy. Springer, 2013, ISBN 978-94-007-5478-2 , p. 218.
- ↑ Victor R. Preedy, Ronald Ross Watson, Vinood B. Patel: Nuts and Seeds. Academic Press, 2011, ISBN 978-0-12-375688-6 , p. 150.
- ↑ J. Schormüller : Alcoholic luxury foods. Handbuch der Lebensmittelchemie, Volume VII, Springer, 1968, ISBN 978-3-642-46131-6 (reprint), p. 611.
- ↑ Maren Kemper: Cutaneous effects of cigarette secondary currents. Dissertation, Universität Hamburg 2007, p. 6, (PDF; 2.3 MB) ( Memento from January 24, 2011 in the Internet Archive )
- ^ Ina-Maria Schneider, Wolfgang Wohlrab, Reinhard Neubert: Fatty acids and epidermis. In: The dermatologist. 48 (5), 1997, pp. 303-310, doi: 10.1007 / s001050050587 .
- ↑ James A. Kent: Handbook of Industrial Chemistry and Biotechnology. Vol. 1, 12th Edition, Springer, 2012, ISBN 978-1-4614-4258-5 , p. 1381.
- ↑ a b entry on lipokine. In: Römpp Online . Georg Thieme Verlag, accessed on October 17, 2017.
- ^ Richard E. Litz: Biotechnology of Fruit and Nut Crops. CABI, 2005, ISBN 0-85199-662-0 , p. 133.
- ↑ Carla Ferreri, Chryssostomos Chatgilialoglu: Membrane Lipidomics. Wiley, 2015, ISBN 978-1-118-54041-1 , pp. 26-30.
- ^ Fatty Acids. Isomers with a mass of 254.1 g mol −1 . In: PlantFA Database. Michigan State University, accessed July 4, 2020 .
- ^ Adam M. Bernstein, Michael F. Roizen, Luis Martinez: Purified palmitoleic acid for the reduction of high-sensitivity C-reactive protein and serum lipids: A double-blinded, randomized, placebo controlled study. In: Journal of Clinical Lipidology. Volume 8, Issue 6, 2014, pp. 612–617, doi: 10.1016 / j.jacl.2014.08.001 .
- ↑ Dairy fat may help not harm In: Harvard Gazette. December 20, 2010.
- ↑ trans fatty acids and their influence on health on dge.de, accessed on October 17, 2017.
- ↑ Not all trans fatty acids are bad on Wissenschaft-aktuell.de, accessed on October 17, 2017.
- ^ Meyer R. Rosen: Delivery System Handbook for Personal Care and Cosmetic Products. William Andrew Pub., 2005, ISBN 0-8155-1504-9 , p. 776.
- ↑ Christos C. Zouboulis, Andreas D. Katsambas, Albert M. Kligman: Pathogenesis and Treatment of Acne and Rosacea. Springer, 2014, ISBN 978-3-540-69374-1 , p. 34 f.
Web links
- Palmitoleic acid - Interesting facts and hit lists (PDF; 158 kB), from archiv.ever.ch, accessed on October 17, 2017 (with a table on the palmitoleic acid content of many foods).

