Cyclopentylamine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
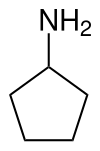
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Cyclopentylamine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 5 H 11 N | ||||||||||||||||||
| Brief description |
colorless to yellowish liquid with an amine-like odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 85.15 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.863 g cm −3 (25 ° C) |
||||||||||||||||||
| Melting point |
-85 ° C |
||||||||||||||||||
| boiling point |
108 ° C |
||||||||||||||||||
| solubility |
miscible with water |
||||||||||||||||||
| Refractive index |
1.4482 |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Cyclopentylamine is an organic-chemical compound from the group of aliphatic primary amines . It is used as an intermediate for the production of active ingredients in pesticides and pharmaceuticals and is sometimes used as a solvent .
Extraction and presentation
Cyclopentylamine is obtained industrially by reductive amination of cyclopentanone with ammonia at temperatures of 150-200 ° C. and pressures around 200 bar in the presence of hydrogen over a nickel contact in a fixed bed reactor .
properties
Physical Properties
Cyclopentylamine has a relative gas density of 2.94 (density ratio to dry air at the same temperature and the same pressure ).
Chemical properties
Cyclopentylamine is a highly flammable, colorless to yellowish liquid with an amine-like odor. The compound belongs to the class of cyclic, primary aliphatic amines. The compound is miscible with water . With acids , strong oxidizing agents , carbon dioxide , acid chlorides and anhydrides , sometimes violent reactions occur.
use
Cyclopentylamine we mainly for the production of plant protection agents such as Pencycuron which as, fungicide for potato and rice plants used is used. It is also used for the synthesis of pharmaceuticals . To some extent it is used directly as a solvent , especially for non-polar substances.
safety instructions
Cyclopentylamine vapors form explosive mixtures with air. The substance is mainly absorbed through the respiratory tract and the skin . Ingestion or exposure leads to acute burns of the mouth , throat and mucous membranes . There is a risk of perforation of the esophagus and stomach . There is also a risk of serious eye damage . Further inhalation consequences are irritation of the mucous membranes, coughing , dyspnoea and damage to the respiratory tract. A mutagenicity could not be detected in the Ames test . A carcinogenicity to humans is not suspected. No detailed information is available on reproductive toxicity . Cyclopentylamine has a lower explosion limit (LEL) of 1.3% by volume and an upper explosion limit (UEL) of 9.4% by volume. The ignition temperature is 260 ° C. The substance therefore falls into temperature class T3. With a flash point of 11.5 ° C, the compound is considered highly flammable. Cyclopentylamine is also classified as hazardous to the aquatic environment.
Web links
Individual evidence
- ↑ a b c d e f g h i Entry on cyclopentylamine in the GESTIS substance database of the IFA , accessed on March 26, 2020(JavaScript required) .
- ↑ a b c Peter Roose, Karsten Eller, Erhard Henkes, Roland Rossbacher, Hartmut Höke: Amines, Aliphatic. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley ‐ VCH Verlag GmbH & Co. KGaA., September 30, 2015, p. 15, doi : 10.1002 / 14356007.a02_001.pub2 .
- ↑ Entry on Cyclopentylamine in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on March 26, 2020. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ a b Cyclopentylamine data sheet from Sigma-Aldrich , accessed on March 26, 2020 ( PDF ).
- ↑ Cyclopentylamine. In: BASF Pharmaceuticals. BASF SE, accessed on March 28, 2020 .


