Dimethylglucamine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Dimethylglucamine | |||||||||||||||
| other names | ||||||||||||||||
| Molecular formula | C 8 H 19 NO 5 | |||||||||||||||
| Brief description |
colorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 209.24 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.1808 g cm −3 at 25 ° C |
|||||||||||||||
| Melting point | ||||||||||||||||
| boiling point |
488.56 K |
|||||||||||||||
| solubility |
soluble in water and isopropanol - acetonitrile mixture |
|||||||||||||||
| Refractive index |
1.4538 (25 ° C, 589 nm) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Dimethylglucamine is a tertiary amine derived from the monosaccharide glucose as a renewable raw material . Because of its basic character, methylmeglumine is used as a low-odor, non-corrosive, environmentally and health-friendly neutralizing agent in water-based emulsion paints and varnishes and in liquid preparations for personal care and cosmetics.
Occurrence and representation
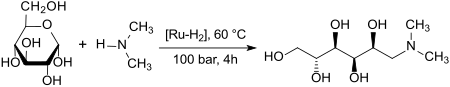
In aqueous solution, D- glucose is reacted with dimethylamine on hydrogenation over a heterogeneous ruthenium catalyst in 95% yield to (in addition to small amounts of sorbitol and N-methyl-D-glucamine ) to dimethylglucamine.
Depending on the reaction temperature and the catalyst / glucose ratio, a retroaldol reaction to the cleavage products dimethylaminoethanol DMAE and tetramethylethylenediamine TMEDA can also take place.
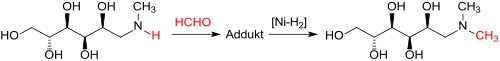
The in as Leuckart reaction known reductive amination of N -methyl- D glucamine (meglumine) - of glucose and methylamine over a ruthenium contact in 96% yield available - with formaldehyde obtained HCHO adduct is of Raney nickel hydrogenated almost quantitatively to methylmeglumine.
Gentle process conditions, such as B. Reaction and hydrogenation at 30 to 40 ° C, followed by post-hydrogenation at 90 ° C, produce instead of brown pale yellow reaction solutions that only contain traces of formaldehyde and the methanol formed therefrom by hydrogenation and are also suitable for cosmetic preparations.
properties
When the aqueous reaction solutions are concentrated, dimethylglucamine is usually obtained as a yellow to brown syrup, which crystallizes out as a colorless solid on cooling. For purification, the raw substance can be recrystallized from ethyl acetate . N, N- dimethyl- D -glucamine is readily soluble in water, so that clear, low-viscosity, light yellow ( Hazen color number <250) 50% aqueous solutions can also be produced. The pH value of a 1% solution is approx. 10.9.
Applications
The aminopolyol N, N -dimethyl- D -glucamine, which is derived in high proportions (approx. 75%) from the renewable raw material sugar, forms with fatty acids such as B. oleic acid , the corresponding ammonium salts , which form homogeneous solutions even at low degrees of neutralization and are characterized by very low viscosities . In this way, highly concentrated liquid detergents and liquid soaps that are easy to handle and can be dosed can be realized.
By quaternizing with longer-chain n- bromoalkanes (C 12 to C 20 ) antimicrobial substances are available in isopropanol-acetonitrile mixtures that are effective against gram-positive bacteria , such as. B. S. aureus , and yeasts such. B. Candida albicans effective against gram-negative bacteria such. B. E. coli, however, are practically ineffective.
Dimethylglucamine is suitable as a low-odor and anti-corrosive substitute for the synthetic pH value regulator aminomethylpropanol (AMP) for water-based varnishes and paints, especially in interior applications. In addition, it increases the thermal stability of the color dispersions and their color strength.
Manufacturer and trade name
Dimethylglucamine managed by Global Amines Germany GmbH, a joint venture of the company Clariant AG and Wilmar International manufactured and marketed under the name Genamin.RTM Gluco 50 technical fields of application or Neutrotain ™ DMG for personal care and cosmetic treatments on the market.
Individual evidence
- ↑ a b c d e F. Gregan, F. Devinsky, J. Zima, D. Mlynarcik, P. Novomesky, I. Lacko, J. Sivy: Synthesis and antimicrobial activity of a series of N, N-dimethyl-N- alkyl-D-glucaminium bromides . In: Chem. Papers . tape 50 , no. 5 , 1996, pp. 310-315 ( chempap.org [PDF]).
- ^ A b c d Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons, 2nd Edition . Elsevier Inc., Oxford, UK 2015, ISBN 978-0-323-28659-6 , pp. 76 .
- ↑ a b Patent EP0614881A1 : Process for the preparation of tertiary dialkyl polyhydroxyamines. Registered on March 1, 1994 , published on September 14, 1994 , applicant: Hoechst AG, inventor: F. Weinelt.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b Patent DE4400591A1 : Process for the production of amino alcohols. Registered on January 12, 1994 , published on July 13, 1995 , applicant: BASF AG, inventor: H.-J. Weyer, HJ Mercker, R. Becker.
- Jump up ↑ J. Poissonnier, M. Pelckmans, F. Van Waes, K. Moonen, BF Sels, JW Thybaut, GB Marin: Kinetics of homogeneous and heterogeneous reactions in the reductive aminolysis of glucose with dimethylamine . In: Appl. Catal. B: Environmental . tape 227 , 2018, p. 161–169 , doi : 10.1016 / j.apcatb.2018.01.025 .
- ↑ Patent WO2018172025A1 : Catalytic process for the production of N, N-dimethylglucamine starting from N-methylglucamine. Registered on February 28, 2018 , published on September 27, 2018 , applicant: Clariant International Ltd., inventors: E. Akgün, M. Link, S. Werner, K. Raab, P. Klug, K. Scheitzeneder, S. Kreuzpointner .
- ↑ Genamin Gluco 50: A multifunctional neutralizer for ecolabel certified paints. (PDF; 5.7 MB) In: clariant.com. Clariant International Ltd., accessed August 11, 2020 .
- ↑ Patent WO2017060481A1 : compositions containing sugar amine and fatty acid. Applied on April 13, 2017 , published on October 7, 2016 , applicant: Clariant International Ltd., inventor: P. Klug, C. Cohrs, U. Back, K. Mutch.