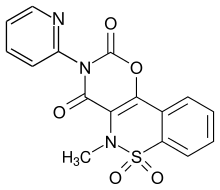
Droxicam
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| General | |||||||||||||
| Non-proprietary name | Droxicam | ||||||||||||
| other names | |||||||||||||
| Molecular formula | C 16 H 11 N 3 O 5 S | ||||||||||||
| Brief description |
white to pale yellow solid |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| Drug class | |||||||||||||
| Mechanism of action | |||||||||||||
| properties | |||||||||||||
| Molar mass | 357.34 g · mol -1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
259-261 ° C |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| Toxicological data |
6192 mg kg −1 ( LD 50 , mouse , oral ) (m)
8841 mg kg −1 ( LD 50 , mouse , oral ) (w)
1434 mg kg −1 ( LD 50 , rat , oral ) (m )
1994 mg kg −1 ( LD 50 , rat , oral ) (w) |
||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Droxicam is a prodrug of piroxicam , which is why it is ascribed similar analgesic , anti-inflammatory and anti-pyretic properties. The Oxicam is used as a non-steroidal anti-inflammatory drug (NSAID).
No preparation with this active ingredient is approved in Germany, Austria or Switzerland.
history
In 1982 Provesan SA registered a patent on Droxicam for the first time. Behind the development were Esteve, Almirall , the Angelini Group and Johnson & Johnson .
The European Committee for Medicinal Products (CPMP) recommends a suspension of approval since December 1994 due to suspicion of more serious hepatoxic side effects than with other approved oxicams - including piroxicam. In some European countries, for example in Spain , it is still available as a medicinal product . In Pakistan it was approved against rheumatoid complaints in 1995.
Extraction
The synthesis from piroxicam with triphosgene results in about 83% droxicam.
Several condensation processes are described in the literature .
properties
Under standard conditions , Droxicam appears as a crystalline powder of pale, light yellow color. The substance is used medicinally because it is metabolized to piroxicam.
pharmacology
Pharmacodynamics
The piroxicam produced during degradation causes a reversible blockade of prostaglandin synthesis by inhibiting cyclooxygenase .
- → See Mechanism of Action of Piroxicam.
Pharmacokinetics
Droxicam is metabolised in the gastrointestinal tract, after oral ingestion the original active ingredient does not appear in the blood. Further metabolism as piroxicam takes place in the liver via cytochrome P450 2C9 . The plasma half-life of the active metabolite is reported to be around 50 hours.
The bioavailability of piroxicam is considered to be almost absolute and does not decrease significantly with simultaneous administration of antacids .
Due to its plasma half-life , which is comparable to piroxicam , it is taken once a day, which means that it reaches steady state after a few days. The usual daily dose is taken in the form of a capsule.
Side effects and contraindications
As with piroxicam, the side effects reported for monopreparations are rash , gastric and duodenal ulcers , edema , hepatitis , cholestasis , colitis , nephritis , malaise, phototoxia , Henoch-Schönlein purpura , hair loss and high blood pressure .
Droxicam is contraindicated for blood formation disorders and gastrointestinal ulcers . The product should not be used during breastfeeding and pregnancy.
An evaluation of national databases for side effects in Spain in 2006 showed increased liver damage in connection with Droxicam.
Trade names
Droxicam is marketed as a medicinal product in only a few countries .
- Human preparations
- Dobenam (I), Drogelon (E), Droxar (I), Droxicam (BG, E), Ferpan (E), Ombolan (AR, BR, E), Pareston (E), Precam
- Chemicals
- AK129765, E-3128
Individual evidence
- ↑ a b Entry on Droxicam at Toronto Research Chemicals , accessed on February 1, 2016 ( PDF ).
- ↑ D07267 at KEGG , accessed in February 2016.
- ↑ Data sheet from CAS number: 90101-16-9 from Santa Cruz Biotechnology, Inc. (English), accessed on February 22, 2016.
- ↑ Pharmaceutical Substance List, 12th Edition 2001, Advertising and Sales Association of German Pharmacists.
- ↑ a b Safety data sheet Arkpharm USA: Droxicam, 98% , accessed on October 11, 2018.
- ↑ a b Droxicam at ChemIDplus (English)
- ↑ a b Derivatives of oxazinobenzothiazine-6,6-dioxide; U.S. Patent # 4563452 (1983)
- ↑ a b Droxicam in ChEBI, accessed on February 21, 2016. (English)
- ↑ Red List Online . Accessed February 2016.
- ↑ Search for ATC M01AC04 in the AGES medicinal specialties register - February 2016.
- ↑ Droxicam in the Swiss Medicines Compendium - February 2016.
- ↑ Patent application FR2528433 : Derivatives of oxazinobenzothiazine-6,6-dioxide. Applied on June 15, 1982 , published on December 16, 1983 , applicant: Provesan SA, inventor: José Esteve Soler.
- ↑ a b c Droxicam at Adis Insight, accessed February 22, 2016.
- ↑ Consolidated List of products whose consumption and / or sale have been banned, withdrawn, severely restricted or not approved by Governments , United Nations, 2003, p. 116 (Edition 2005) (English) , accessed on February 16, 2016.
- ↑ Search M01AC04 at ChemInfo Withdrawn Drugs, accessed on February 23 2016th
- ^ F1 Pérez-Aguilar, Berenguer M, Ramírez-Palanca JJ, Payà A, Vera-Sempere FJ, Sánchez-Cuenca JM, Berenguer J .: [Chronic autoimmune hepatitis following cholestatic hepatitis caused by droxicam] . In: Med Clin (Barc). . 106, No. 12, March 30, 1996, pp. 460-2. PMID 8656732 .
- ^ A b M1 Lapeyre-Mestre, AM de Castro, MP Bareille, JG Del Pozo, AA Requejo, LM Arias, JL Montastruc, Carv: Non-steroidal anti-inflammatory drug-related hepatic damage in France and Spain: analysis from national spontaneous reporting systems. . In: Fundam Clin Pharmacol . 20, No. 4, August 2006, pp. 391-5. PMID 16867024 .
- ^ Franz Bracher, Caroline Berns: Triphosgene in Heterocyclic Chemistry: A novel synthesis of the antiinflammatory prodrug droxicam . In: Journal for practical chemistry / Chemiker-Zeitung . tape 339 , no. 1 , January 1997, p. 477-478 , doi : 10.1002 / prac.19973390185 .
- ^ Pharmaceutical Substances, Axel Kleemann, Jürgen Engel, Bernd Kutscher and Dietmar Reichert, 4th edition (2000), Thieme-Verlag Stuttgart, ISBN 978-1-58890-031-9 . P. 723.
- ^ J1 Esteve, Farré AJ, Roser R .: Pharmacological profile of droxicam . In: General Pharmacology . 19, No. 1, 1988, pp. 49-54. PMID 3278945 .
- ↑ L. Martínez, J. Sánchez: Pharmacokinetic profile of droxicam . In: European Journal of Rheumatology and Inflammation . tape 11 , no. 4 , January 1991, pp. 10-14 , PMID 1365484 .
- ↑ MT1 Maya, Pais JP, Ruas Da Silva J, Morais JA .: A comparative bioavailability study to estimate the influence of an antacid on droxicam pharmacokinetics . In: European Journal of Drug Metabolism Pharmacokinetics . 20, No. 4, Oct-Dec 1995, pp. 275-9. PMID 8983932 .
- ↑ Ombolan capsules, accessed February 2016.
- ↑ Swiss Pharmacists' Association (ed.): Index nominum 2000: international drug directory . 17th edition. Medpharm Scientific Publ, Stuttgart 2000, ISBN 3-88763-075-0 , p. 377 .
- ↑ Droxicam at aptekt.framar.bg (Bulgarian) , accessed February 2016.
