1,8-cineole
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
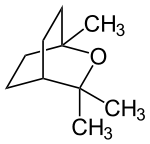
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 1,8-cineole | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 10 H 18 O | ||||||||||||||||||
| Brief description |
colorless to yellowish, camphor-like smelling liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 154.24 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.93 g cm −3 |
||||||||||||||||||
| Melting point |
1.5 ° C |
||||||||||||||||||
| boiling point |
174-177 ° C |
||||||||||||||||||
| Vapor pressure |
1.22 h Pa (20 ° C) |
||||||||||||||||||
| solubility |
poor in water (3.25 g l −1 at 21 ° C) |
||||||||||||||||||
| Refractive index |
1.4586 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
1,8-Cineol belongs to the bicyclic epoxy monoterpenes , more precisely the limonene oxides . The organic compound is a colorless liquid. It is used for respiratory diseases such as bronchitis or colds of the airways, but also for chronic and inflammatory respiratory diseases as well as asthma and hay fever.
Occurrence

1,8-cineole occurs in larger amounts in eucalyptus ( eucalyptus oil contains approximately 85 percent cineole) and bay leaf . It is less abundantly present in mint , medicinal sage , cannabis , thyme , basil and in the tea tree .
biosynthesis
For the biosynthesis of 1,8-cineol in the plant, the dimethylallyl pyrophosphate (DMAPP) is necessary as a starter, which is combined with the isomeric isopentenyl pyrophosphate , which comes from the pyruvate-glyceraldehyde phosphate path ("Rohmer path") Tail reaction condensed.
Extraction
Cineole can be obtained in large quantities by the fractional distillation of eucalyptus oil. At a purity of 99.6 from 99.8 percent one speaks of technical cineole.
properties
1,8-cineole smells fresh and camphor-like . It is miscible with ether , ethanol and chloroform in any ratio. The flash point is 49 ° C.
Analytics
The coupling of capillary gas chromatography with mass spectrometry after appropriate sample preparation of the test material is suitable for reliable qualitative and quantitative determination of 1,8-cineole . This analytical procedure is also suitable for differentiating eucalyptus oils of different species.
use
1,8-cineole is used on the one hand for respiratory diseases in humans, but mainly in veterinary medicine . On the other hand, it is used as a flavoring in the perfume industry . In dentistry it is used for the revision of root fillings.
Pharmacological effects
1,8-Cineol has an expectorant and bactericidal effect in the lungs and sinuses in humans . It also inhibits certain neurotransmitters that are responsible for narrowing the bronchi. In asthmatics , the lung function can be improved under medical supervision by administration of pure cineole. However, cineol is only an alternative to corticosteroids in exceptional cases, which can be used locally as inhalation and with few side effects. Even in the chronic obstructive pulmonary disease COPD , pure cineole as an additional medication to standard therapy can under certain circumstances improve lung function and thus reduce exacerbations .
The known side effects of 1,8-cineole are slight liquefaction of stool and possibly slight nausea . Both occur only when taken orally . Furthermore, allergic reactions have also been described, especially in children. It is taken by oral administration of capsules, which only dissolve in the small intestine , by inhalation or by preparing corresponding plants containing the active ingredient as an infusion .
Trade names
Soledum (D), Sinolpan Forte (D)
GeloMyrtol (D), Rowachol (D, A), Rowatinex (D, A), Transpulmin (D)
Web links
- More about cineole: Use as a medicinal plant
Individual evidence
- ↑ Entry on EUCALYPTOL in the CosIng database of the EU Commission, accessed on February 25, 2020.
- ↑ a b c d e f g Data sheet 1,8-Cineol (PDF) from Merck , accessed on May 25, 2011.
- ↑ a b c Entry on 1,8-cineole in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-256.
- ↑ M. Rohmer: The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. In: Nat Prod Rep . 16, 1999, pp. 565-574.
- ↑ NH El-Soud, M. Deabes, LA El-Kassem, M. Khalil: Chemical Composition and Antifungal Activity of Ocimum basilicum L. Essential Oil. In: Open Access Maced J Med Sci. 3 (3), Sep 15, 2015, pp. 374-379. PMID 27275253
- ↑ AF Blank, LC CAMELO, F. Arrigoni-Blank Mde, JB Pinheiro, TM Andrade, S. Niculau Edo, PB Alves: Chemical Diversity in Lippia alba (Mill.) NE Brown Germplasm. In: ScientificWorldJournal. 2015, p. 321924. PMID 26075292
- ↑ PC Buenoa, MG Junior, JK Bastos: A GC-FID validated method for the quality control of Eucalyptus globulus raw material and its pharmaceutical products, and gc-ms fingerprinting of 12 Eucalyptus species. In: Nat Prod Commun. 9 (12), Dec 2014, pp. 1787-1790. PMID 25632486
- ↑ K. Sebei, F. Sakouhi, W. Herchi, ML Khouja, S. Boukhchina: Chemical composition and antibacterial activities of seven Eucalyptus species essential oils leaves. In: Biol Res. 48, Jan 19, 2015, p. 7. PMID 25654423
- ↑ a b Red List online, as of October 2009.
- ↑ UR Juergens, U. Dethlefsen, G. Steinkamp, A. Gillissen, R. Repges, H. Vetter: Anti-inflammatory activity of 1.8-cineol (eucalyptol) in bronchial asthma: a double-blind placebo-controlled trial. In: Respir Med. 97 (3), Mar 2003, pp. 250-256. PMID 12645832
- ↑ H. Worth include: Concomitant therapy with Cineole (Eucalyptole) Reduces exacerbations in COPD: A placebo-controlled double-blind trial. In: Respir Res. 10 (1), 2009, p. 69.
- ↑ Peter Altmeyer, Martina Bacharach-Buhles: Springer Encyclopedia Dermatology, Allergology, Environmental Medicine. Springer 2002, ISBN 978-3-540-41361-5 , p. 302. Limited preview in the Google book search.
- ↑ AGES-PharmMed, as of October 2009.

