History of fuel cells
The history of fuel cells describes the discovery, research and development of the various fuel cells and their corresponding systems from the discovery of the functional principle by Schönbein and Grove in 1838 and 1839 to modern technical applications such as the fuel cell vehicle .
prehistory
In 1800, Alessandro Volta sent a letter to London with a report on his voltaic column . This first modern battery was the starting point for a lively research activity that led to improved batteries, but also to the discovery of electrolysis . As early as 1800, William Cruickshank, William Nicholson and Anthony Carlisle discovered water electrolysis , the breakdown of water into hydrogen and oxygen.
In 1838 William Robert Grove worked intensively on optimizing conventional batteries. He also discovered a cell with an amalgamated zinc electrode on the one hand and a platinum electrode in nitric acid on the other hand, which delivered a voltage of almost 2 V and was therefore superior to contemporary cells. Through his later work, is generally considered to be the inventor of the fuel cell.
1838 to 1843: Discovery of the "gas battery"
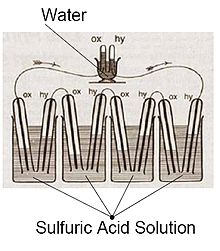
However, Christian Friedrich Schönbein was the first to contribute to the discovery and described the first experiment in which a voltage was determined between two electrodes in an aqueous solution that were wetted with hydrogen and oxygen. In December 1838 he described in his publication that platinum electrodes in sulfuric acid are charged in opposite directions, depending on whether hydrogen or oxygen (or chlorine) is present. With that he had found the principle of the fuel cell.
Then William Robert Grove also built a corresponding voltage source. As he reported in January 1839, he was able to convert hydrogen and oxygen on electrodes made of platinum wires and thus obtain an electrical voltage by reversing the electrolysis of water. Then he combined such cells to form a battery, which he called a “gas battery” - the term fuel cell did not exist at the time. In 1842/1843 he presented improved cells that contained platinum- coated platinum foil strips and, due to the enlarged and catalytically active surface, were stronger than those with smooth electrodes.
1874 to 1950: patents and laboratory research on the "fuel cell"
In 1874 Grove confirmed Schönbein's observation that the "gas battery" does not only work with hydrogen and oxygen. He reported that the combination of hydrogen and chlorine made a very powerful fuel cell. Instead of hydrogen, it could also have a weak effect with ethene .
In 1889 the chemists Carl (Charles) Langer and Ludwig Mond developed an improved design. They used a hydrogen-containing mixture made from coal, air and water as fuel gas, the moon gas , and also coined the term "fuel cell".
The physical chemist Wilhelm Ostwald reported in 1894 that fuel cells, unlike internal combustion engines, are not subject to the limited efficiency of heat engines . He therefore saw electrochemistry as the way to efficient energy conversion. Ostwald had also specifically suggested coal as a fuel, whereupon researchers and inventors tried to implement the concept. In 1896 , the US inventor William W. Jacques applied for a patent for a carbon fuel cell . However, it did not bring the hoped-for success: the efficiency of 82% given by the inventor is said to have been only 8% according to his critics' calculations.
For an improved conversion of hydrogen, Alfred Schmid (1899–1968) described a gas diffusion electrode with a large inner surface that delivered very strong currents in 1923 and 1924 in little-noticed papers .
Walther Nernst had a patent on the in 1897 Nernst received. In 1900 this was brought onto the market, which showed the suitability of solid electrolytes for technical applications. Building on this, Emil Baur , William Treadwell (patents 1919 and 1920) and Hans Preis designed and tested the first fuel cells with solid electrolyte , which could be operated at temperatures of up to 1000 ° C. OK Davtyan further developed solid electrolytes and corresponding cells in Moscow in the 1940s . He published the first book on fuel cells in 1947.
The English engineer Francis Bacon (1904–1992) worked on hydrogen-oxygen fuel cells with alkaline electrolytes since the late 1930s. From 1940 he worked at King's College in Cambridge. He developed the first practically usable systems, for which he proposed an application in submarines. He also received appropriate support from government agencies.
From around 1950: industrial research and development for application readiness
In 1959, after two decades of development work, Francis Bacon presented a system with an output of up to 5 kW. He was able to show that a combination of pressure tanks and fuel cells was superior to a battery if a considerable amount of electrical power had to be provided over a long period of time. In the same year, the first fuel cell vehicle , the Allis Chalmers fuel cell tractor , was presented. In 1961 a cell was described with a liquid solution of methanol as fuel and a hydrogen peroxide solution as oxidant, which could deliver an output of 600 watts. Another cell for liquid fuels developed at the time could be operated with ethylene glycol , which at the time was, however, too expensive for technical use.
In 1966 General Motors presented the first fuel cell car , the Electrovan, but it remained a demonstration object.
During the oil price crisis of 1973 the demand for alternative fuels was very high. The increasingly strict emission laws, especially in California, ensured that the electric drive became more and more popular as an alternative. Although these had some advantages over fossil fuels, mainly due to the efficient, quiet and emission-free work, they were still at a disadvantage in terms of weight, price, charging time and range. Despite this, some automobile manufacturers brought battery-powered cars onto the market.
At the beginning of the nineties, the fuel cell was considered as an alternative, although at the time it was mainly used in space travel as an efficient source of energy and was not yet suitable for the operation of a conventional car. However, Daimler researchers teamed up with Ballard Power Systems and developed a fuel cell system suitable for vehicles. The result of the collaboration was presented in 1994.
Overall, the advantages of avoiding harmful exhaust gases and noise levels were already known, but the high costs of providing hydrogen and the lack of practicable alternatives prevented a forced development for commercial use. In addition, the required battery mass was still unrealistically large: With the Bacon system , a conceivable fuel cell city bus would have required a battery weighing around 14 tonnes, and the service life of systems at that time was still very limited. In 1962, the Justi engine was rated as the most promising for use in road vehicles, the battery mass of which would have been only 5 tons for a city bus. The lowach fuel cell was named as another promising principle for road vehicles, which would later be used not only in space travel, but also in today's road vehicles. Other fuel cell systems were developed by Kordesch among others at this time .
Application in space
Several generations of fuel cells were developed and used for the US space program: a 1.0 kW system in the Gemini program and a 1.5 kW system in the lunar flight project ( Apollo program ). An important advantage of the hydrogen-oxygen fuel cell was that the cell also provided drinking water for the crews. The system of manned Gemini orbiting the earth was based on the principle of the polymer electrolyte fuel cell, which was developed by General Electric with the assistance of Leonard Niederach . The polymer electrolytes consisted of solid ion exchange resins made from sulfonated polystyrene . The equipment for the Apollo lunar program was supplied by Pratt and Whitney . The entire system weighed 810 kg and contained 31 individual cells connected in series, which delivered up to 500 kWh. They were alkaline cells which contained 75% potassium hydroxide and which were operated at over 200 ° C. The space shuttle contained three units, each weighing just over a hundred pounds. Together they provide an average of 7 kW and a maximum of 12 kW. The practical application of the fuel cell in space travel was possible due to the provision of extraordinarily high funding, but it was still far from being economically viable and therefore initially not generally transferable.
On the way to the practical fuel cell road vehicle
The Canadian company Ballard Power Systems , founded in 1979, has been researching fuel cells since 1983, initially with support from the Canadian Ministry of Defense . A high-performance cell stack with twelve cells had been built by 1986. In 1993 the first demonstration fuel cell bus was completed. Also in 1993, Daimler-Benz and Ballard signed a contract to develop road vehicles. Then in 1994 Daimler presented the NECAR 1 (New Electric Car), which was based on the Mercedes-Benz MB 100 van .

In 1996 the NECAR 2 followed, also a transporter, this time a converted Mercedes-Benz W 638 . This offered space for six passengers and had a range of 250 km. In 1997 Daimler-Benz bought 25% of the shares in Ballard, Ford another 15%. In addition, the NECAR 3 was presented in 1997, a modified Mercedes-Benz A-Class with a top speed of 120 km / h. The fuel cells were operated with hydrogen like the previous ones, but this was generated in a methanol reformer while driving . The NECAR 4 from 1999, which was filled with liquid hydrogen, already had a range of 450 kilometers.
21st century: attempts and successes in commercialization
In the years from 2000 to 2010, many prototypes of small fuel cells for applications in mobile electronics were demonstrated. Firms announcing devices with direct methanol fuel cells included Sony, Toshiba, NEC, Fujitsu, and Motorola. Others, e.g. B. Casio, combined a small methanol reformer with a hydrogen fuel cell (PEM). Hardly any of these devices were actually marketed. One reason for this is likely to be the falling price and increasing performance of lithium-ion batteries.
SFC Energy , founded in 2000, sells various direct methanol fuel cells - both devices for special and industrial applications as well as those for end customers, e.g. B. for motorhomes. According to its own information, it had sold over 41,000 fuel cells by January 2019.
The German Howaldtswerke- of the shipyard Kiel (HDW) developed and since 2003 built submarines of class 212 and class 214 are driven by air-independent fuel cell systems.
For the supply of households with electricity and heat, cogeneration fuel cell systems are increasingly being offered that can be operated with hydrogen, natural gas or methane.
Several car manufacturers produced fuel cell vehicles in small series: Hyundai has been manufacturing the Hyundai ix35 FCEV since 2013 . Toyota has been selling the Mirai since 2014 and Honda has been supplying the Honda FCX Clarity since 2008 . According to the US Environmental Protection Agency (EPA), the improved version of the Clarity, which will also be sold in Europe from the end of 2016, has the highest range of all-electric vehicles at 589 km.
Individual evidence
- ^ William Robert Grove: LVI. On a new voltaic combination . In: David Brewster, Richard Taylor, Richard Phillips (Eds.): Philosophical Magazine - The London and Edinburgh Philosophical Magazine and Jornal Of Science . Series 3rd volume 13 , no. 84 . Richard and John E. Taylor, 1838, ISSN 0031-8086 , LVI, p. 430–431 , doi : 10.1080 / 14786443808649618 (English, google.de [accessed on November 11, 2016] Grove separates the electrodes of its galvanic cells, for example by a plate made of unglazed, porous porcelain. Such a diaphragm allows the Different solutions can be used at different poles of the battery. Here he only uses copper sulfate solutions and iron sheets.): “the porous filter as […] an important element”
- ^ A b William Robert Grove: On voltaic series and the combination of gases by platinum . Swanson, Dec. 14, 1838. In: David Brewster, Richard Taylor, Richard Phillips (Eds.): Philosophical Magazine - The London and Edinburgh Philosophical Magazine and Jornal Of Science . Series 3rd volume 14 , no. 86 . Richard and John E. Taylor, 1839, ISSN 1941-5966 , XXIV, p. 127–130 , doi : 10.1080 / 14786443908649684 (English, archive.org [accessed on November 11, 2016] In the main part of the work (submitted on December 14, 1839), various combinations of electrolyte solutions are examined with regard to their effect in galvanic cells. Only in the appendix added later (January 1839) does he talk about the gas electrodes. Here he only uses a single cell, not yet a series connection, ie no battery.): “Strips of platinum […] hermetically sealed, through the bottom of a bell glass; […] The glass was filled with water acidulated with sulfuric acid […] one of oxygen, the other of hydrogen ”
- ^ John Meurig Thomas: WR Grove and the fuel cell . In: Philosophical Magazine . tape 92 , no. 31 . Taylor & Francis, November 2012, ISSN 1478-6443 , pp. 3757–3765 , doi : 10.1080 / 14786435.2012.691216 (English): “Grove assembled a powerful source of energy in which Pt in the O 2 of one pair was metallically connected with the Pt in the H 2 of the next”
- ^ A b Gerd Sandstede, Elton J. Cairns, VS Bagotsky, K. Wiesener: History of low temperature fuel cells . In: Wolf Vielstich, Arnold Lamm, Hubert A. Gasteiger (Ed.): Handbook of Fuel Cells . John Wiley & Sons, Ltd, Chichester, UK 2010, ISBN 978-0-470-74151-1 , chap. 12 , p. 145-218 , doi : 10.1002 / 9780470974001.f104011 ( wiley.com ).
- ↑ Christian Friedrich Schönbein: New observations on the voltaic polarization of solid and liquid conductors . In: Johann Christian Poggendorff (Ed.): Annals of Physics and Chemistry . Second row. tape 17 , no. 1 . Johann Ambrosius Barth, Leipzig 1839, V, p. 101–123 ( online at Gallica Bibliotèque nationale de France [accessed on November 22, 2016] Basel, December 1938. See in particular page 105, section 10): “If water (made more conductive by a little sulfuric acid) is shaken with hydrogen gas, this liquid in a glass tube connected at the bottom with a bubble, [...] so you get a current ... "
- ^ Christian Friedrich Schönbein: On the voltaic polarization of certain solid and fluid substances . In: David Brewster, Richard Taylor, Richard Phillips (Eds.): Philosophical Magazine - The London and Edinburgh Philosophical Magazine and Jornal Of Science . Series 3rd volume 14 , no. 85 . Richard and John E. Taylor, 1839, ISSN 0031-8086 , X, p. 43–45 , doi : 10.1080 / 14786443908649658 (English, google.de [accessed on November 11, 2016] The background to the work is the question of how the voltage arises in galvanic elements. The argument makes use of the catalytic effect of platinum, which was already known at the time the conversion of hydrogen and oxygen.): “water slightly acidulated with sulphuric acid and holding some hydrogen dissolved […] the current in question is caused by the combination of hydrogen with (the) oxygen (contained dissolved in water)”
- ^ William Robert Grove: On the Gas Voltaic Battery. Experiments Made with a View of Ascertaining the Rationale of Its Action and Its Application to Eudiometry . In: Royal Society (Ed.): Philosophical Transactions of the Royal Society of London . For the year 1843. Volume 133 , no. 2 . Richard and John E. Taylor, 1843, ISSN 0261-0523 , VIII, p. 91–112 , doi : 10.1098 / rstl.1843.0009 , JSTOR : 108377 (English, google.de [accessed on November 11, 2016] the same also in: The London, Edinburgh and Dublin Philosophical Magazine and Journal of Science): “strips of well-platinized platinum foil ”
- ^ A b William Robert Grove: The correlation of physical forces . Longmans, Green and Co., 1874, Gas Voltaic Battery, p. 271–274 (English): “Chlorine and hydrogen gave very powerful effects […] This is the most powerful gas battery […] olefiant gas, which apperas to give rise to acontinuous thoughe extremely feeble current”
- ^ Friedrich Wilhelm Ostwald: The scientific electrochemistry of the present and the technical of the future . In: Journal for electrical engineering and electrochemistry . today: Reports from the Bunsen Society for Physical Chemistry. tape 1 , no. 4 , July 15, 1894, ISSN 0005-9021 , p. 122–125 , doi : 10.1002 / bbpc.18940010403 ( first page at the publisher - the direct implementation of coal in a fuel cell that Ostwald dreamed of is conceivable, but far from what is possible today.): “Now the path on which this largest of all technical There are questions to be solved, the procurement of cheap energy, this path must be found by electrochemistry. If we have a galvanic element which directly supplies electrical energy from coal and the oxygen in the air, in an amount that is somewhat in proportion to the theoretical value, then we are facing a technical revolution, against which the invention of the Steam engine has to disappear. "
- ^ Friedrich Wilhelm Ostwald: The scientific electrochemistry of the present and the technical of the future . Lecture given to the 2nd annual meeting of the Association of German Electrical Engineers on June 8, 1894 in Leipzig. In: Wilhelm Ostwald and J. H. van't Hoff (ed.): Journal for physical chemistry, stoichiometry and kinship theory . tape 15 , no. 4 . Wilhelm Engelmann, Leipzig 1894, p. 409-421 ( online at Internet Archive [accessed November 11, 2016]).
- ↑ Patent US555511 : Method of converting potential energy from carbon into electrical energy. Published March 3, 1896 , inventor: William W. Jacques.
- ↑ Matthew Brown, Matthew Cohen, Keith Gary: Fuel Cell Origins. William Jacques' carbon battery apparatus, 1896. In: Fuel Cells. Smithsonian Institution, 2001, accessed on May 20, 2018 (English): "The result was an efficiency of only 8 percent."
- ↑ Werner Schnurnberger: Fuel Cell Technology Handbook. In: Book Reviews. pro-physik.de, Wiley-VCH Verlag, accessed on November 16, 2016 .
- ↑ Alfred Schmid : The diffusion gas electrode . Ferdinand Enke, Stuttgart 1923, DNB 362325200 , OCLC 41832578 .
- ↑ Alfred Schmid: The diffusion gas electrode . In: Helvetica Chimica Acta . tape 7 , no. 1 , 1924, ISSN 1522-2675 , pp. 370–373 , doi : 10.1002 / hlca.19240070143 : "The effectiveness of the hydrogen electrode is very bad, since the hydrogen is only active where the three phases: electrolyte-gas-metal come together at the same time."
- ↑ Patent GB126766 : Improvements in Electric Cells or Batteries. Applied on March 16, 1918 , published on May 16, 1919 , inventors: Emil Baur, William Dupré Treadwell (coke electrode, iron oxide catalyst for oxygen reduction).
- ↑ Patent DE325783 : fuel element . Registered on September 20, 2016 , published on September 17, 1920 , inventor: Emil Baur, William Dupré Treadwell ("To burn coal with an electric motor [...] the fuel electrode-forming coal (coke, hard coal, etc.)").
- ^ Emil Baur, Hans Price: About fuel chains with fixed ladders . In: Journal of Electrochemistry and Applied Physical Chemistry . Reports of the Bunsen Society for Physical Chemistry. tape 43 , no. September 9 , 1937, ISSN 0005-9021 , pp. 727-732 , doi : 10.1002 / bbpc.19370430903 .
- ↑ V. Yu. Baklan, Oleksandr D. Vasylyev, Valeriy S. Kublanovskii, Boris M. Grafov: To the centenary of OK Davtyan (April 15, 1911 – December 1, 1990) . In: Russian Journal of Electrochemistry . tape 48 , no. 3 . Pleiades Publishing, Springer, March 2012, ISSN 1023-1935 , p. 348-349 , doi : 10.1134 / S1023193512660019 ( springer.com ).
- ↑ Bent Sörensen; Michael A. Priestnall, Vega P. Kotzeva, J. Fish, Eva M. Nilsson: Fuel Cells Compendium . Hydrogen and Fuel Cells. Eds: Nigel P. Brandon, David Thompsett. Elsevier, Amsterdam a. a. 2005, ISBN 0-08-044696-5 , Chapter 30. Compact mixed-reactant fuel cells, p. 595 (English, google.de [accessed on November 18, 2016] accordingly the cell was described in: PG Grimes, B. Fielder, J. Adam 1961 Proc. Annu. Power Sources Conf. 15 (1961) 29-32).
- ↑ The fuel cell as an energy source for road vehicles that do not depend on overhead contact lines. In Automobiltechnik 10/1962, pp. 403-405.
- ^ Region Stuttgart and the fuel cell alliance Baden-Württemberg: The fuel cell in the region Stuttgart Analysis and expansion of the value chain. In: http://www.bba-bw.de . Stuttgart Region and the Fuel Cell Alliance Baden-Württemberg, January 13, 2019, accessed on January 13, 2019 (German).
- ↑ The fuel cell as an energy source for road vehicles that do not depend on overhead contact lines. In Automobiltechnik 10/1962 , pp. 403-405.
- ^ A b c d e Marvin Warshay, Paul R. Prokopius: The fuel cell in space: Yesterday, today and tomorrow . Grove Anniversary Fuel Cell Symposium. In: NASA Lewis Research Center (Ed.): NASA Technical Report . tape 102366 . Cleveland, Ohio 1989 (English, 10 pages, nasa.gov [PDF; 507 kB ; retrieved on November 11, 2016]): “Noteworthy among these advantages was the ability of the hydrogen-oxygen fuel cell to supply potable water”
- ↑ a b c Ballard Power Systems Inc. (pdf) The Power to Change the World. In: The Practice of Innovation - Seven Canadian Firms in Profile. Government of Canada Publications, 2003, pp. 20–31 , accessed on December 9, 2016 (English): “the company has been working on refining fuel cell technology for commercial uses since 1983. […] in 1986, Ballard Power Systems reached a breakthrough point. "
- ^ NECAR: New Electric Car. In: History of Fuel Cell Vehicles. dieBrennstoffzelle.de, accessed on December 9, 2016 : "a maximum speed of 110 km / h and a tank range of 250 km"
- ↑ Xianglin Li, Amir Faghri: Review and advances of direct methanol fuel cells (DMFCs) part I: Design, fabrication, and testing with high concentration methanol solutions . In: Journal of Power Sources . tape 226 . Elsevier BV, 2013, 3. Advances in DMFC prototype designs and developments, 3.2. DMFC stack development using high concentration methanol, p. 223–240 , doi : 10.1016 / j.jpowsour.2012.10.061 (English, researchgate.net [PDF; accessed on November 11, 2016]): “The state-of-the-art DMFC prototypes and products are more competitive than rechargeable batteries, especially in applications such as military uses. "
- ↑ Casio's new device slashes size of PC fuel cells . In: Fuel Cells Bulletin . To International Newsletter. tape 2006 , no. 5 . Elsevier Lt, May 2006, ISSN 1464-2859 , p. 1 , doi : 10.1016 / S1464-2859 (06) 71030-6 (English, els-cdn.com [PDF; accessed on November 11, 2016] announced a runtime that is four times longer than that of lithium-ion batteries of the same size): "The new reformer will be able to run for 20 h on a single charge"
- ↑ Archived copy ( memento of the original dated November 19, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ SFC Energy receives follow-up order from the Bundeswehr for a portable SFC energy network with fuel cells. In: Investors, News, Press Release. SFC Energy AG, January 22, 2019, accessed on April 23, 2019 .
- ↑ ASUE - Working Group for Economical and Environmentally Friendly Energy Consumption eV (Ed.): Fuel cells for domestic energy supply . Functionality, development and market overview. March 2016 ( asue.de [PDF; 5.0 MB ; accessed on November 11, 2016]): "The fuel cell is currently in the market launch phase"
- ↑ John Voelcker: 2017 Honda Clarity Fuel Cell rated at 366 miles of range by EPA. Green Car Reports, October 24, 2016, accessed November 16, 2016 .