Guanylurea
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Guanylurea | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 2 H 6 N 4 O | ||||||||||||||||||
| Brief description |
white solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 102.10 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
105 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
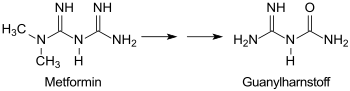
Guanylurea is a product of the reaction of dicyandiamide DCD with strong acids such as B. sulfuric acid H 2 SO 4 to guanylurea sulfate, and is formed by subsequent release of the base , z. B. by means of barium hydroxide . 1-Carbamoylguanidine is the main breakdown product of the anti-diabetic drug metformin and a starting material for active pharmaceutical ingredients, pesticides and explosives.
Occurrence and representation
Guanyl urea does not occur naturally, but is found widespread in surface waters in concentrations of up to 28 µg / l as a breakdown product of the biguanide metformin, which is effective against type 2 diabetes . The active ingredient is not metabolized in the human organism and about 90% excreted unchanged, but anaerobically degraded to guanylurea in wastewater treatment plants .
In the Ruhr in 2018, GU was the second most common among the regularly measured trace substances after the complexing agent EDTA with 3.3 tons per year - but with a decreasing tendency for years (in 2016: 5.6 to / a). With adapted activated sludge , however, guanyl urea can also be completely broken down.
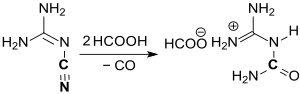
In the chemical synthesis of guanylurea (referred to here as dicyandiamidine), first reported in 1862, dicyandiamide is converted into a dilute mineral acid, such as B. nitric acid , hydrochloric acid or sulfuric acid or a strong organic acid, such as. B. formic acid , where on heating to 80 ° C cyanoguanidine DCD dissolves and reacts in an exothermic reaction with hydrolysis of the cyano group to the amide group to form the guanylurea salt of the corresponding acid.
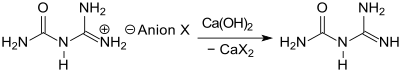
The readily water-soluble salts of guanylurea are only obtained when cooling "in a high stage of purity and very good yields" if the reaction temperature is kept at approx. 100 ° C and the amount of water is limited, or dissolved salt with a water-miscible organic solvent such as Acetone is precipitated. The base guanylurea can be released from the aqueous solution of guanylurea sulfate by adding barium hydroxide , and from other salts by means of calcium hydroxide at temperatures <50 ° C.
properties
1-Carbamoylguanidine is a white solid that crystallizes in “long, glass-shining prisms” and dissolves well in water and pyridine and in ethanol when heated . Because of its lower thermal stability - the nitrate and perchlorate salts are resistant up to> 200 ° C - and the pronounced hygroscopicity and adsorption of CO 2 from the air, the base guanylurea is usually in the form of its water-soluble mineral salts, such as. B. nitrate, chloride , phosphate or sulfate or their soluble in dipolar aprotic solvents organic salts such. B. the format is used.
Applications
Guanylurea nitrate GUN forms a gas-generating complex with basic copper (II) nitrate BCN as an oxidizing agent, which is proposed as a substitute for poisonous sodium azide as a gas generator for airbags - as well as so-called "high-energy" salts of the GU, e.g. B. azide anions.
The salt guanylurea dinitramide GUDN, which is accessible from guanidine carboxamide and ammonium dinitramide, is being investigated as a halogen-free, “green” propellant for rocket engines and, because of its extremely high insensitivity to impact and friction, as a robust explosive.
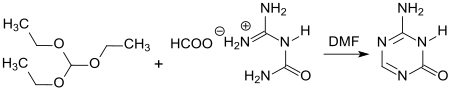
5-Azacytosine is a 1,3,5-triazine ( sym -triazine) in which the 5-CH group of the nucleic base cytosine has been replaced by a nitrogen atom. It is produced in a yield of approx. 80% in the reaction of guanyl urea or guanyl urea formate with triethyl orthoformate HC (OEt) 3 in dimethylformamide DMF at approx. 100 ° C.
The cytostatics azacitidine (5-azacytidine) and decitabine (5-aza-2'-deoxycytidine) are synthetic nucleosides , both of which have 5-azacytosine as a nucleic base.
Individual evidence
- ↑ a b c d Entry on guanylurea at Toronto Research Chemicals , accessed on May 20, 2020 ( PDF ).
- ↑ a b c J. Söll, A. Stutzer: Communications about some new compounds that are obtained from guanylurea and from diguanide . In: Ber. German chem. Ges. Band 42 , no. 4 , 1909, pp. 4532-4541 , doi : 10.1002 / cber.19090420453 .
- ↑ a b David R. Lide: CRC Handbook of Chemistry and Physics A Ready-reference Book of Chemical and Physical Data . CRC Press, 1995, ISBN 978-0-8493-0595-5 , pp. 22 ( limited preview in Google Book search).
- ↑ a b J. Haag: About dicyandiamide and a new base resulting from it . In: Liebigs Ann. Chem. Band 122 , no. 1 , 1862, p. 22-33 , doi : 10.1002 / jlac.1862220103 .
- ^ S. Tisler, C. Zwiener: Formation and occurrence of transformation products of metformin in wastewater and surface water . In: Sci. Total Environ. tape 628–629 , 2018, pp. 1121–1129 , doi : 10.1016 / j.scitotenv.2018.02.105 .
- ↑ M. Scheurer, A. Michel, H.-J. Brauch, W. Ruck, F. Sacher: Occurrence and fate of the antidiabetic drug metformin and its metabolite guanylurea in the environment and during drinking water treatment . In: Water Res. Band 45 , no. 15 , 2012, p. 4790-4802 , doi : 10.1016 / j.watres.2012.06.019 .
- ↑ Ruhr Quality Report 2018. Ruhrverband, 2019, pp. 86–87 , accessed on May 21, 2020 .
- ↑ S. Tisler, C. Zwiener: Aerobic and anaerobic formation and biodegradation of guanyl urea and other transformation products of metformin . In: Water Res. Band 45 , no. 149 , 2019, pp. 130–135 , doi : 10.1016 / j.watres.2018.11.001 .
- ↑ a b c Patent US2277823 : Preparation of guanyl urea. Filed on December 13, 1939 , published on March 31, 1942 , Applicant: American Cyanamid Co., Inventor: GH Foster, DW Jayne, Jr
- ↑ Patent CA427499A : Guanyl urea format production. Published on 15 May 1945 , Applicant: American Cyanamid Co., Inventor: HM Day, DW Jayne, Jr
- ↑ RC Hartenstein, I. Fridovich: Amidinourea formats, a precursor of 2-amino-4-hydroxy-s-triazine . In: J. Org. Chem. Band 32 , no. 5 , 1967, p. 1653-1654 , doi : 10.1021 / jo01280a095 .
- ↑ Thomas M. Klapötke : The preparation and characterization of guanylurea nitrate and perchlorate salts . In: Heteroatom Chem. Volume 19 , no. 3 , 2008, p. 301-306 , doi : 10.1002 / hc.20419 .
- ↑ Patent EP1335890B1 : Gas generation with metal complexes of guanyl urea nitrate. Filed November 15, 2001 , published September 29, 2004 , applicant: Autoliv ASP, Inc., inventor: IV Mendenhall.
- ^ TM Klapötke, CM Sabaté: Low energy monopropellants based on the guanylurea cation . In: ZAAC . tape 636 , no. 1 , 2010, p. 163-175 , doi : 10.1002 / zaac.200900330 .
- ↑ Patent WO2010017374A1 : Process for preparing azacitidine intermediate. Registered on August 6, 2009 , published on February 11, 2010 , applicant: Sicor Inc., inventors: E. Bigatti, G. Lux, M. Paiocchi, A. Giolito, S. Tosi.
- ↑ A. Piskalá: Nucleic acid components and Their analogues. CI. Synthesis of 5-azacytosine (4-amino-1,2-dihydro-1,3,5-triazin-2-ones) and its methyl derivatives . In: Collect. Czech. Chem. Commun. tape 32 , no. 11 , 1967, p. 3966-3976 , doi : 10.1135 / cccc.19673966 .