Japanese encephalitis
| Classification according to ICD-10 | |
|---|---|
| A83.0 | Japanese encephalitis |
| ICD-10 online (WHO version 2019) | |
The Japanese Encephalitis (abbreviation: PER ), and encephalitis japonica , is a by viruses triggered tropical disease that especially in East and Southeast Asia occurs. In the endemic areas , 30,000–50,000 people fall ill each year, especially children. Adults are mostly immune.
Pathogen
| Japanese encephalitis virus | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Systematics | ||||||||||||||||||
|
||||||||||||||||||
| Taxonomic characteristics | ||||||||||||||||||
|
||||||||||||||||||
| Scientific name | ||||||||||||||||||
| Japanese encephalitis virus | ||||||||||||||||||
| Short name | ||||||||||||||||||
| JEV | ||||||||||||||||||
| Left | ||||||||||||||||||
|
Japanese encephalitis , also Japanese B encephalitis or Russian autumn (al) encephalitis, is caused by the Japanese encephalitis virus ( scientifically Japanese encephalitis virus , JEV), an arbovirus (arthropod-borne virus) that, like the causative agent of yellow fever (scientific yellow fever virus , YFV) and dengue fever (scientific dengue virus , DENV) belongs to the Flaviviridae (genus Flavivirus ). As of September 2019, five genotypes of the virus had been identified (JAOARS982, M28, Nakayama, SA (V) and SA-14).
infection
Japanese encephalitis is a zoonosis - the pathogen reservoir ( main hosts , reservoir hosts ) is made up of pigs and wild birds (especially herons and other waders ), and more rarely horses , reptiles and bats . The vectors ( vector ) are mosquitoes of the genera Culex , Aedes and possibly others. The most important are Culex tritaeniorhynchus (rice field mosquito ) and Culex vishnui.
The risk of infection for tourists is very low (<1 / million). There is an increased risk of infection with long-term stays in the endemic areas , especially in the countryside, especially at the end of the rainy season in the temperate areas and all year round in the tropics. The risk of infection is clearly linked to rice cultivation and pig breeding.
Epidemiology
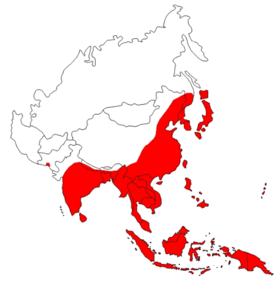
Japanese encephalitis is widespread in Asia ; around three billion people live here in YEN endemic areas . In Japan itself, due to the systematic vaccination of domestic animals, only a few cases occur. Mainly affected are China , India , Sri Lanka , Nepal , Vietnam , the Philippines and northern Thailand . Annually, 35,000 to 50,000 cases with more than 10,000 deaths are known in the endemic areas, whereby the actual number of diseases is likely to be significantly higher.
Symptoms
In most cases, the infection is mild or even asymptomatic . 1 out of 250 sufferers develop a severe course with inflammation of the brain ( encephalitis ). After an incubation period of 5 to 15 days, sudden fever , chills , headache and muscle pain ( myalgia ) occur. Vomiting and / or diarrhea often occur in children. Disturbances in consciousness occur within a short time. Various neurological symptoms can occur. The mortality rate when the disease breaks out is high (5–30%) and the disease often leaves permanent damage.
Diagnosis
With appropriate exposure , the suspected diagnosis can be made from the clinical picture. Further diagnostics include:
- Serological antibody detection (IgM capture ELISA test, virus detection using PCR is only possible in the first week of illness)
- Blood count: leukocytosis
- CT or MRI
- CSF examination : lymphocytic pleocytosis with normal glucose level
Differential diagnoses include cerebral malaria , bacterial meningitis , other viral infections such as enterovirus 71 or others.
therapy
As there are currently no specific effective drugs against JE, therapy is symptomatic and limited to symptom relief . It includes the support of vital functions (circulation, respiration) and the prevention of secondary infections .
prevention
The most important preventive measure is to avoid mosquito bites at dusk and at night. Tourists should protect themselves with repellants , mosquito nets, and covering clothing. If you stay in endemic areas for a longer period of time, vaccination against JE is recommended, which offers very good protection after two injections (day 0 and day 28). The duration of the protective effect is not yet known; the WHO currently recommends booster vaccinations after 3 years.
vaccination
So far, two vaccines of Japanese origin (mainly Je-Vax® Biken) have been used worldwide , while China has used Chinese products. A new inactivated (non-live) vaccine with the strain SA-14-14-2 IXIARO® from Valneva (formerly Intercell) has been available in the USA and Europe since 2009. The vaccine virus is grown on Vero cells and no longer in mouse brains, as with the older vaccines. The neutralizing antibodies that are formed and are a measure of protection are as good as the old vaccines. IXIARO is approved for active immunization against the Japanese Encephalitis Virus in adults, adolescents, children and infants from the age of 2 months and is administered intramuscularly in the deltoid muscle of the upper arm (infants can also be vaccinated in the anterolateral thigh muscles). The basic vaccination consists of two doses of vaccine 28 days apart; it should be completed at least 1 week before exposure . Another vaccination after 1–2 years is recommended. The reported adverse effects of IXIARO are minor, most commonly local pain, headache, muscle pain, fatigue. The dreaded reactions of the old vaccines (neurological and allergic) have not yet been determined. Because the number of people vaccinated so far is still small, rare undesirable side effects cannot be ruled out. In addition to water , additives in IXIARO are NaCl , disodium hydrogen phosphate, potassium dihydrogen phosphate and possibly traces of protamine sulfate and formaldehyde from the manufacturing process. It does not contain thimerosal , gelatine or other stabilizers and preservatives. In China, a live vaccine with the strain SA 14-14-2 is used and used in large vaccination campaigns with good success. A chimeric vaccine ChimeriVax-Je® will soon be approved in Australia and Thailand . DNA vaccines are in development, but not yet ready for the market.
Indications for vaccination
The World Health Organization (WHO) recommends the vaccination for travelers to Asia who stay for a long time in countries where Japanese encephalitis occurs more frequently. These are Bangladesh , China , India , Indonesia , Japan , Cambodia , North and South Korea , Laos , Myanmar , Nepal , parts of Oceania , the Philippines , Indus Delta of Pakistan , Sri Lanka , Taiwan , Thailand and Vietnam . In particular, rural regions are affected in mosquito-rich seasons.
Contraindications (contraindication) to vaccination
People with acute illnesses requiring treatment with a high fever should not be vaccinated. Also, people with known or suspected severe hypersensitivity to components of the vaccine used, such as people who have had an allergic reaction, high fever or other adverse reaction to this vaccine, must not be vaccinated. These include an itchy rash all over the body, a severely swollen face or swelling, shortness of breath and water retention also on the arms, legs or neck.
The benefits and risks of this vaccination must be carefully weighed for the following people:
- Pregnant and breastfeeding women
- People who have ever suffered from an allergy or nettle rash
- People with immune disorders
literature
- Scott B. Halstead, Julie Jacobson: Japanese encephalitis. In: Advances in Virus Research. Vol. 61, 2003, ISSN 0065-3527 , pp. 103-138, doi : 10.1016 / S0065-3527 (03) 61003-1 .
- Ernest A. Gould, T. Solomon: Pathogenic flaviviruses. In: The Lancet . Vol. 371, No. 9611, 2008, pp. 500-509, doi : 10.1016 / S0140-6736 (08) 60238-X .
- Scott B. Halstead, Julie Jacobson: Japanese encephalitis vaccines. In: Stanley A. Plotkin, Walter A. Orenstein, Paul A. Offit Vaccines. 5th edition. Saunders / Elsevier, Philadelphia PA 2008, ISBN 978-1-416-03611-1 .
- Marc Fischer, Nicole Lindsey, J. Erin Staples, Susan Hills: Japanese Encephalitis Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP). In: Morbidity and Mortality Weekly Report. Recommendations and Reports. Vol. 59, No. RR-1, March 12, 2010, ISSN 1057-5987 , pp. 1–32, ( digital version (PDF; 1.55 MB) ).
- Susan L. Hills, Anne C. Griggs, Marc Fischer: Japanese Encephalitis among travelers from non endemic countries, 1973-2008. In: The American Journal of Tropical Medicine and Hygiene. Vol. 82, No. 5, 2010, ISSN 0002-9637 , pp. 930-936, doi : 10.4269 / ajtmh.2010.09-0676 .
Web links
- Robert Koch Institute : Profiles of Rare and Imported Infectious Diseases PDF , September 15, 2011, p. 24.
Individual evidence
- ↑ ICTV Master Species List 2018b.v2 . MSL # 34, March 2019
- ↑ a b c d ICTV: ICTV Taxonomy history: Yellow fever virus , EC 51, Berlin, Germany, July 2019; Email ratification March 2020 (MSL # 35)
- ↑ DDr. Martin Haditsch - New vaccines against meningococcal meningitis and Japanese encephalitis . Foreign Office website. Retrieved January 28, 2014.
- ^ World Health Organization : Japanese encephalitis