Laurylamine dipropylenediamine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Laurylamine dipropylenediamine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 18 H 41 N 3 | ||||||||||||||||||
| Brief description |
clear colorless to pale yellow liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 299.54 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.865 g cm −3 at 20 ° C |
||||||||||||||||||
| Melting point |
9 ° C |
||||||||||||||||||
| boiling point |
182-184 ° C (1 torr) |
||||||||||||||||||
| solubility |
soluble in water and in methanol |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Laurylamine dipropylenediamine is a derivative of dodecylamine , which is formed by cyanoethylation with acrylonitrile and subsequent catalytic hydrogenation . The long-chain triamine has surfactant and biocide properties and is used in lubricating oils and cooling lubricants as a corrosion protection agent and in disinfectant cleaners .
Occurrence and representation
The industrial production of N , N -Bis (3-aminopropyl) dodecylamine starts from the saturated linear C 12 fatty acid lauric acid [C 11 H 23 -COOH], which with ammonia (NH 3 ) to form the lauric acid amide [C 11 H 23 - CONH 2 ], and then reacted for Laurinsäurenitril (Dodecannitril, C 11 H 23 -CN) is dehydrated is.
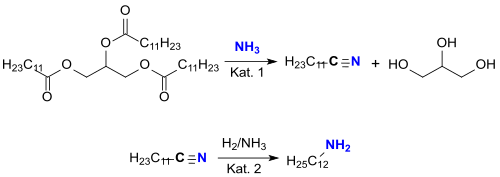
Recently, lauric acid-rich vegetable oils , such as. B. coconut oil (a triglyceride mixture with about 50% lauric acid), in the vapor phase with ammonia on transition metal catalysts such. B. vanadium (V) oxide V 2 O 5 or zeolites , such as. B. HZSM-5 (Cat. 1) converted directly at 400 ° C into a fatty acid nitrile mixture rich in laurine nitrile (and glycerine ), from which the dodecanenitrile can be distilled out.
The laurine nitrile is hydrogenated to laurylamine on a Raney nickel catalyst (cat. 2) in the presence of ammonia to suppress the formation of secondary amines.
Dodecylamine adds at 110 ° C to excess acrylonitrile in the sense of a Michael addition . This results in N, N -Di (cyanoethyl) dodecylamine, which in the next step with Raney cobalt at 140 ° C in the presence of ammonia in 88% yield to N - (3-aminopropyl) - N -dodecylpropane-1,3- diamine is hydrogenated.
With Raney nickel as the catalyst, laurylamine dipropylenediamine is obtained practically quantitatively even at a low hydrogen pressure of about 3.5 bar.
properties
Laurylamine dipropylenediamine is a clear, colorless to pale yellow and low viscosity (38 m Pa · s ) liquid with an amine-like odor, the aqueous solutions of which are strongly alkaline (pH 11.2 in 1% solution at 20 ° C). As a surfactant, DPTA shows strong foaming and is compatible with nonionic , cationic and some anionic surfactants . The compound is biodegradable (96% in 12 days).
Applications
Laurylamine dipropylenediamine is a pH-stable surfactant with biocidal properties against bacteria, including mycobacteria and problem germs such as. B. pseudomonads , and against enveloped viruses , such as. B. the influenza virus influenza A virus H1N1 and the hepatitis B virus (HBV), also in the presence of blood and proteins. The compound is therefore used as a disinfectant for instruments in medicine and dentistry and for germ reduction on solid surfaces, e.g. B. used in the food industry.
Because of its germ-inhibiting effect, DPTA is also used in closed water cycles, such as in the paper industry and in cooling towers, and for the preservation of cooling lubricants such as drilling and cutting oils, where its dispersant , lubricant and corrosion protection properties are also useful.
Laurylamine dipropylenediamine is manufactured and marketed by Lonza AG under the brand name Lonzabac® 12 and by Nouryon, formerly AkzoNobel , under the brand name Triameen® Y12D.
Individual evidence
- ↑ Entry on LAURYLAMINE DIPROPYLENEDIAMINE in the CosIng database of the EU Commission, accessed on March 13, 2020.
- ↑ a b c d e Entry on N- (3-aminopropyl) -N-dodecylpropane-1,3-diamine in the GESTIS substance database of the IFA , accessed on February 19, 2020 (JavaScript required)
- ↑ a b c d e Worksafe Australia (Ed.): Full Public Report: Lonzabac 12.100, File No: NA / 64 . Sydney December 16, 1992 ( gov.au ).
- ↑ Frost, Albert E., Jr .; Martell, Arthur E .: Preparation of trimethylenediamines . In: Journal of Organic Chemistry . tape 15 , 1950, pp. 51-53 , doi : 10.1021 / jo01147a009 .
- ^ A b T. T. Denton, AS Joyce, DE Kiely: Preparation of N -alkylbis (3-aminopropyl) amines by the catalytic hydrogenation of N -alkylbis (cyanoethyl) amines . In: J. Org. Chem. Band 72 , no. 13 , 2007, p. 4997-5000 , doi : 10.1021 / jo070245v .
- ↑ Z. Shirazi, H. Tafazolian, S. Viamajala, S. Varanasi, Z. Song, MJ Heben: High-yield production of fatty nitriles by one-step vapor-phase thermocatalysis of triglycerides . In: ACS Omega . tape 2 , no. 12 , 2017, p. 9013-9020 , doi : 10.1021 / acsomega.zb01502 .
- ↑ H. Oskarsson, M. Frankenberg, A. Annerling, K. Holmberg: Adsorption of novel alkylaminoamide sugar surfactants at tailor-made surfaces . In: J. Surfact. Chem. Band 10 , 2007, p. 41-52 , doi : 10.1007 / s11743-006-1007-1 .
- ↑ a b Lonza, Hygiene Product Catalog. (PDF; 410 KB) In: www.lonza.com/hygiene. Lonza AG, accessed on March 10, 2020 (English).
- ↑ Patent US6610248B1 : Disinfectants. Registered on June 23, 2000 , published on August 26, 2003 , applicant: Lonza AG, inventor: F. Lichtenberg, M. Lützeler, V. Ranft.
- ↑ Triameen Y12D. (PDF; 210 KB) In: www.surfacechemistry.nouryon.com. Nouryon, accessed March 10, 2020 .