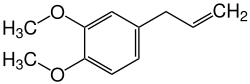
Methyl eugenol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Methyl eugenol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 11 H 14 O 2 | ||||||||||||||||||
| Brief description |
colorless liquid with a pleasant odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 178.23 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.04 g cm −3 |
||||||||||||||||||
| Melting point |
−4 ° C |
||||||||||||||||||
| boiling point |
|
||||||||||||||||||
| Vapor pressure |
<1 h Pa (20 ° C) |
||||||||||||||||||
| solubility |
practically insoluble in water (0.5 g · l −1 ) and glycerine , soluble in various oils |
||||||||||||||||||
| Refractive index |
1.50 |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Methyleugenol is a natural product. It belongs to a group of lipophilic phenylpropanoids with an allylic side chain which, according to animal experiments , can be carcinogenic and mutagenic when taken orally in higher concentrations .
Occurrence and manufacture
Methyleugenol is a natural component - often together with eugenol - of essential oils made from fennel , rose , basil , anise , allspice , nutmeg , bay or laurel . It is also found in other essential oils , including pine oil and cinnamon oil . Synthetic methyl eugenol is made from eugenol by methylation .
Metabolism
safety instructions
Since the substance has often been found in tea infusions, the Federal Institute for Risk Assessment in 2001 indicated the urgency that methyleugenol must not be detectable in such tea products - especially since these drinks are often given to infants and young children. In the Flavor Ordinance for Food and the German Cosmetics Ordinance , a ban on the use of the substance methyl eugenol has since been established. Methyl eugenol may not be used in the manufacture or treatment of cosmetic products. Exceptions are normal levels in the natural essential oils used, provided that the concentration does not exceed the following values:
- 0.01% in perfume
- 0.004% in eau de toilette
- 0.002% in cream perfume
- 0.001% in products to be rinsed out / rinsed off
- 0.0002% in other products that remain on the skin / hair and oral products.
In 2008, the Federal Institute for Consumer Health Protection and Veterinary Medicine (BgVV) found increased concentrations in cosmetics containing tea tree oil . The IARC classified methyl eugenol as a possible carcinogen in 2013.
Web links
- Federal Institute for Risk Assessment: Information on Methyleugenol
Individual evidence
- ↑ a b c d e f g h i Entry on methyleugenol in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ a b c data sheet methyl eugenol at Good Scents Company (English).
- ↑ GESTIS note: The manufacturer's classification does not take into account the opinion of the European Commission "Health and Consumer Protection" (PDF; 34 kB). According to this, the substance is carcinogenic and should be labeled with H350.
- ↑ a b entry on eugenol methyl ether. In: Römpp Online . Georg Thieme Verlag, accessed on July 24, 2011.
- ^ Contents of methyl eugenol and estragole in tea-like products (PDF; 35 kB) BgVV opinion of November 12, 2001.
- ↑ Regulation (EC) No. 1223/2009 of the European Parliament and of the Council of November 30, 2009 on cosmetic products (PDF; 3.3 MB), accessed on March 2, 2017 .
- ↑ 7th meeting of the BfR Commission for Cosmetic Products, minutes of the meeting on May 19, 2011 (PDF; 72 kB) Item 7: Essential oils / tea tree oil, p. 4f.
- ↑ IARC Monograph 101 - Methyleugenol, 2013


