Multiphoton microscope

A multiphoton microscope ( English Multi-Photon Laser Scanning Microscope , MPLSM, also multi-photon microscopy , MPM) is a special light microscope from the group of laser scanning microscopes .
Images are created using one of two different physical phenomena:
- Multiphoton fluorescence (mostly two-photon fluorescence) or
- Higher Harmonic Generation ( doubling ( Second Harmonic Generation , SHG) or tripling ( Third Harmonic Generation , THG) of the oscillation frequency of the radiated light).
With the help of a strong, focused laser beam , non-linear optical effects are generated that are based on the interaction of several photons (light particles) arriving at the same time in a molecule . The strength of the generated signal therefore does not increase linearly with the number of irradiated photons, but with the square (with two-photon effects) or the third power (with three-photon effects).
The operation of a multiphoton microscope is similar to that of a confocal laser scanning microscope . However, while confocal laser scanning microscopy has a penetration depth of 50–80 µm, depending on the specimen, multi-photon microscopy allows deeper areas, e.g. B. of 200 microns, in very favorable cases even up to 1000 microns (= 1 mm) can be achieved. This enables recordings of living tissues that are otherwise inaccessible for imaging.
Multi-photon fluorescence microscopy
The most widespread multiphoton technique is two-photon fluorescence microscopy, sometimes just called two-photon microscopy. In the conventional fluorescence microscopy in an becomes fluorescent molecule, an electron respectively by the absorption of a photon excited , that is placed in a higher energy state. In two-photon fluorescence microscopy, the electron is excited by the simultaneous absorption of two photons ( two-photon absorption ). Excitation with three or more photons arriving at the same time is also possible.
principle

Fluorescence occurs when dyes absorb incoming photons and then emit another photon. The incoming, "stimulating" photon lifts an electron to a higher energy level, which means that the energy is temporarily stored. In normal fluorescence microscopy, this excitation occurs through exactly one photon. The electron remains at the higher energy level for a few nanoseconds before falling back again and emitting a new, longer-wave, lower-energy photon. When excited with blue light, for example, usually green fluorescence occurs, for example with fluorescein .
This single excitation photon can be replaced by two or more photons if they have the same energy in total. For example, dark red or infrared light can be used to generate green fluorescence. In addition, both photons must arrive at the same time (within one attosecond = 10 −18 s), since there is no stable intermediate energy level .
In normal fluorescence microscopy, the exciting photon has a shorter wavelength , i.e. H. a higher frequency and thus more energy than the emitted photon. The difference in wavelengths between the two photons is called the Stokes shift . In contrast to this, with multi-photon excitation, excitation is carried out with photons that have a significantly longer wavelength, lower frequency and thus less energy per photon than the emitted photons. This is only possible because here two or more stimulating photons lead to the generation of only one emitted photon. In the case of two-photon excitation, the excitation wavelength is approximately twice the normally used excitation wavelength, and in the case of three-photon excitation it is three times.
Technical implementation
In order to achieve a simultaneous arrival of two or more photons at the excitable electrons in the focus point , very high photon densities are required. These are only achieved if a pulsed laser with mode locking is used. The special thing about this type of laser is that very short (e.g. 0.14 ps = 0.14 · 10 −12 s), intense laser pulses are emitted. B. repeated 80 million times per second. In the example given, the pauses between two pulses are 12.5 ns (= 12,500 ps) long, so that all of the energy generated in the laser can be released in a fraction of the time.
The titanium: sapphire lasers usually used for excitation are expensive (approx. 150,000 euros) and therefore represent a major hurdle for widespread use. Ti: Sa lasers can be set to wavelengths from approx. 700 nm to approx. 1050 nm become. Larger wavelengths can be generated by using an " optical parametric oscillator " (OPO). This is "pumped" with the Ti: Sa laser and can then generate wavelengths of over 1300 nm. This means that red and dark red fluorescent dyes can also be excited in the two-photon mode.
As with a confocal laser scanning microscope , the laser beam is focused on a point on the specimen through the objective of the microscope. The position of the laser beam is changed by moving mirrors located in the beam path (scan mirror; English to scan = to scan ) so that the focal point moves through the specimen, i.e. scans it. The resulting fluorescence is captured by the lens, spectrally separated by dichroic beam splitters and sent to the detector (mainly photomultiplier , but also avalanche photodiodes ). The detectors measure the brightness of each pixel one after the other . At no point in time is a complete image of the specimen created in the microscope; this is only put together in the control computer.
Due to their complexity, complete devices are currently only offered by a few manufacturers. In Europe these include Carl Zeiss , LaVision BioTec , Leica Microsystems , Nikon , Olympus , JenLab GmbH and Till Photonics . Since the scanning and detector technology of a multiphoton microscope is very similar to that of a confocal laser scanning microscope, there are some companies that offer corresponding extensions, as well as working groups that upgrade such microscopy to a multiphoton microscope with a suitable excitation laser and the appropriate filters.
advantages
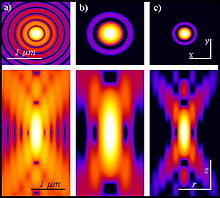
As shown above, the generation of the two-photon effect requires a very high photon density, which only a pulsed laser can achieve. But even in this case there is only a sufficiently high photon density in the focal point to generate fluorescence excitation, but not above and below it (see figure): Outside the focal plane, the same amount of excitation photons is distributed over a rapidly increasing diameter of the beam cone. Two-photon excitation, however, depends on the square of the light intensity, so that the light intensities outside the focal plane, in contrast to other fluorescence microscopes, are no longer sufficient to generate fluorescence.
This has practical advantages:
- A fading of fluorescent dyes and the generation of phototoxicity is limited to an extremely small area of the focal point. Levels above and below are not affected.
- All of the fluorescence captured by the objective can be used for the image to be created. In contrast to the confocal laser scanning microscope, no pinhole is necessary to filter out light from other levels. Therefore, again in comparison to the confocal laser scanning microscope, it is also not necessary to collect the fluorescence via the scanning mirror. Instead, a “ non-descanned detection ” can be carried out. The detection can thus take place spatially closer to the specimen, which in turn allows the collection of part of the fluorescence scattered in the specimen.
- An independent advantage is the greater penetration depth due to the lower scattering of longer-wave light. The scattering cross-section σ depends very strongly on the frequency ν and increases proportionally to ν 4 . Short-wave violet light (400 nm) has a frequency twice as high as long-wave red light (800 nm) and is therefore 16 times more scattered ( see The blue or the red of the sky ). Wavelength-dependent scattering also occurs in biological tissues : when a hand held a powerful flashlight is used, almost only the red portion of the light penetrates. Since infrared or dark red light is used for fluorescence excitation in two-photon microscopy, correspondingly deeper regions can be reached.
Higher Harmonic Generation
In addition to fluorescence, the second and third harmonic generation (SHG or THG; literally: generation of the second (or third) harmonic; in German also: frequency doubling or frequency tripling ) play a role in multiphoton microscopy . They are summarized as the Higher Harmonic Generation (HHG).
This generation of lower wavelength light is not physically related to multiphoton fluorescence excitation. However, HHG occurs under the same lighting conditions as multiphoton fluorescence, namely (only) with very strong excitation light. HHG signals are therefore only generated in the focal point of a pulsed laser, but not above or below it. The sections 'Technical Implementation' and 'Advantages' shown above apply accordingly. The necessary technical equipment is largely the same, so that, for example, a device that was built for two-photon fluorescence microscopy usually also enables second harmonic generation .
Basics
Light is electromagnetic radiation , so it has an electric field . This field interacts with the irradiated matter. When the pulsed laser is focused on a specimen in the multiphoton microscope, these interactions lead to the creation of " harmonics ". The wavelength of the "Second Harmonic" is exactly half of the radiated light, that of the " Third Harmonic " at THG is exactly one third.
In contrast to fluorescence, no energy remains in the preparation with HHG, and the signal does not fade either. However, phototoxic effects can arise independently of the HHG effect through the simultaneous excitation of auto-fluorescence or through absorption. Too high an intensity of the excitation light can also lead directly to the destruction of specimens.
Second Harmonic Generation
The generation of an SHG signal, i.e. frequency doubling, is only possible if the electrical properties of the irradiated molecule differ in all spatial directions, i.e. if it is asymmetrical, more precisely non- centrosymmetrical .
The SHG signal mainly propagates in the "forward" direction, just like the incoming light beam: The individual phases of the forward-directed photons (the SHG signal) are mostly in phase ( coherent ), so that the waves coming from different molecules are generated, amplify. In other directions, the waves partially cancel each other out ( destructive interference ). The proportions of the forward and backward directed signal also depend on the structure of the irradiated molecules. The strength of the resulting signal is also dependent on the direction of polarization of the incoming laser light. In the case of objects with a longitudinal structure (e.g. muscle fibers), this results in a dependence of the signal strength on the orientation of the polarization plane of the laser in relation to the specimen.
The mechanism of formation means that SHG is generated particularly efficiently on some periodic structures, e.g. B. urea crystals . In biological tissues, it arises on collagen fibers and on myosin in smooth muscles . In this way, SHG facilitates orientation in the specimen, even if mainly fluorescence is to be observed.
Since the short-wave light that can be recorded on a microscope is usually in the blue range, a wavelength of over 800 nm is used to generate SHG signals.
Third harmonic generation
If the excitation wavelength is above 1200 nm, Third Harmonic Generation (THG; frequency tripling) can also be observed or captured with filters for visible light. In contrast to SHG, THG does not depend on the existence of non-centrosymmetrical structures. With a sufficiently high intensity of the incident light, THG can in principle be produced in every substance, but the strength of the signal depends on the material (see frequency doubling ). High-contrast GHG images are created when structures of different optical density lie next to one another, for example cells and blood plasma.
History and Applications
1931-1990
The physical principle of fluorescence excitation of a molecule by several photons was first predicted in 1931 by Maria Goeppert-Mayer . The first experimental observation of two-photon fluorescence excitation was made in 1961, soon after the development of the first laser. Microscopy with two-photon fluorescence excitation was successful for the first time in 1990.
The SHG effect was observed immediately after the laser was developed in 1960. Microscopic it was first used in 1974, initially in a conventional light microscope without scanning technique: Robert Hellwarth and Paul Christensen ( University of Southern California to the structure of, Los Angeles) zinc selenide - polycrystals to investigate. In 1978, SHG was first created with a scanning microscope by JN Gannaway and CJR Sheppard. They were the first to be able to restrict the generated signal to the focal plane. They also examined crystals. The continuously radiating lasers used in this process release so much energy in the specimen that biological specimens are destroyed. This problem was only solved with the introduction of pulsed lasers, because only then is the average energy input sufficiently low.
Probably the first application of SHG to a biological preparation was achieved in 1980 by Isaac Freund's group at Bar-Ilan University in Ramat-Gan , Israel , when they examined collagen in the tendons of rats . An nd: YAG laser with a fixed wavelength of 1064 nm was used and the signal was collected in the forward direction. Initially, the intensity could be measured as a function of the angle of incidence of the laser beam, but an image could not yet be created. The same working group succeeded in doing this on the same object in 1986.
Since 1990
After Denk et al. After demonstrating two-photon fluorescence microscopy for the first time in 1990, it took only four more years to succeed in performing it on live animals ( intravital microscopy ), in this case to study blood flow and the behavior of white blood cells in the kidney.
The advantages of a multiphoton microscope, especially the high penetration depth, come into play particularly in tissues where there are structural differences between the upper and deeper tissue layers: The deeper tissue layers are not accessible for other types of microscopy or are only accessible in fixed, cut specimens. Processes there cannot therefore be observed in any other way in living organs. Examples are live examinations in different layers of the brain , the observation of different cells of the immune system in lymph nodes , examinations of how tumor cells can invade neighboring tissues and examinations of muscle cells in the intact heart. In the examples mentioned, two-photon fluorescence and in some cases also SHG were used.
While the observation of fluorescence in flat specimens (individual cells, tissue sections) can also take place well in normal fluorescence microscopes or in confocal laser scanning microscopes, HHG is only possible with a multiphoton microscope. In addition to the collagen fibers and muscle myosin already mentioned, starch and, to a lesser extent, cellulose also lead to SHG.
It is also possible to use specific dyes that cause SHG and, for example, stain biomembranes. In 1996, SHG was published on living cells for the first time using such a dye, which is sensitive to the membrane potential. In the case of other SHG membrane dyes, the SHG signal is lost when two membranes marked in this way come together, as a centrosymmetry suddenly occurs. This process can therefore be determined very sensitively.
SHG microscopy suffered for a long time from very long recording times of minutes to hours per image. This only changed in 1999 when it was possible to generate SHG on a laser scanning microscope equipped with a Ti: Sa laser.
GHG has been used in only a few published biomedical studies to date. One reason for this is that with conventional titanium: sapphire lasers, which are usually used for multiphoton microscopes, the maximum available wavelength (below 1100 nm) is not sufficient to generate GHG in the visible range. However, UV light cannot be absorbed with the usual equipment, so other excitation lasers are used for GHG. Previous applications were, for example, the observation of fat droplets or the observation of hydroxyapatite crystals in tooth enamel . With an excitation wavelength above 1200 nm, the observation of THG signals in the blue and SHG signals in the red part of the spectrum is possible. This was used, for example, to display intact mouse embryos in three dimensions.
Web links
- A direct look into the brain: two-photon microscopy is revolutionizing brain research . In: Scinexx - The Knowledge Magazine.
- Multiphoton Fluorescence Microscopy. In: Microscopy Primer. Florida State University(English).
- Multiple-photon excitation fluorescence microscopy. University of Wisconsin (English).
- Fundamentals and Applications in Multiphoton Excitation Microscopy . Nikon MicroscopyU (English).
- More light! The microscope of the future (German) .
Individual evidence
- ^ A b C. Sumen, TR Mempel, IB Mazo, UH von Andrian: Intravital microscopy: visualizing immunity in context . In: Immunity . tape 21 , no. 3 , September 2004, p. 315-329 , doi : 10.1016 / j.immuni.2004.08.006 , PMID 15357943 .
- ↑ a b P. Friedl, AT den Boer, M. Gunzer: Tuning immune responses: diversity and adaptation of the immunological synapse . In: Nat. Rev. Immunol. tape 5 , no. 7 , June 2005, p. 532-545 , doi : 10.1038 / nri1647 , PMID 15999094 .
- ↑ Patrick Theer, Mazahir T. Hasan, Winfried Denk: Two-photon imaging to a depth of 1000 µm in living brains by use of a Ti: Al 2 O 3 regenerative amplifier . In: Optics Letters . tape 28 , no. 12 , 2003, p. 1022-1024 ( abstract ).
- ↑ a b c d e f Alberto Diaspro, Paolo Bianchini, Giuseppe Vicidomini, Mario Faretta, Paola Ramoino, Cesare Usai: Multi-photon excitation microscopy . In: BioMedical Engineering OnLine . tape 5 , no. 1 , 2006, p. 36 , doi : 10.1186 / 1475-925X-5-36 , PMID 16756664 , PMC 1550243 (free full text).
- ↑ a b c d W. Denk, DW Piston, WW Webb: Multi-Photon Molecular Excitation in Laser-Scanning Microscopy. In: James B. Pawley (Ed.): Handbook of biological Confocal Microscopy. 3. Edition. Springer, New York NY 2006, ISBN 0-387-25921-X .
- ↑ Data sheet of the Chameleon Ultra II (PDF; 363 kB) from Coherent .
- ↑ Barry R. Masters: Confocal Microscopy And Multiphoton Excitation Microscopy: The Genesis of Live Cell Imaging . SPIE Press, Bellingham, Washington 2006, ISBN 978-0-8194-6118-6 , Chapter 12: Theory and Instrumentation of Multiphoton Excitation Microscopy, pp. 169-177 .
- ↑ Peter TC So, Chen Y. Dong, Barry R. Masters, Keith M. Berland: Two-photon excitation fluorescence microscopy . (PDF; 4.7 MB). In: Annu. Rev. Biomed. Closely. No. 2, 2000, pp. 399-429.
- ↑ a b c d e Peter Friedl, Katarina Wolf, Gregory Harms, Ulrich H. von Andrian: Biological second and third harmonic generation microscopy . In: Curr Protoc Cell Biol . Chapter 4, March 2007, Unit 4.15 , doi : 10.1002 / 0471143030.cb0415s34 , PMID 18228516 .
- ^ A b c d e Guy Cox, Eleanor Kable: Second-Harmonic Imaging of Collagen . In: Cell Imaging Techniques . Humana Press, Totowa NJ 2006, ISBN 1-58829-157-X , pp. 15-35 , doi : 10.1007 / 978-1-59259-993-6_2 .
- ↑ a b c d Guy Cox: Optical Imaging Techniques in Cell Biology . CRC Press, Taylor & Francis Group, Boca Raton, FL 2007, ISBN 978-0-8493-3919-6 , Chapter 8: Nonlinear Microscopy, pp. 101-114 .
- ↑ M. Göppert-Mayer: About elementary acts with two quantum leaps . In: Ann Phys . tape 9 , 1931, pp. 273-295 , doi : 10.1002 / andp.19314010303 .
- ↑ a b W. Denk, JH Strickler, WW Webb: Two-photon laser scanning fluorescence microscopy . In: Science (journal) . tape 248 , no. 4951 , April 1990, pp. 73-76 , doi : 10.1126 / science.2321027 , PMID 2321027 .
- ^ R. Hellwarth, P. Christensen: Nonlinear optical microscopic examination of structure in polycrystalline ZnSe . In: Optics Communications . tape 12 , no. 3 , November 1974, p. 318-322 , doi : 10.1016 / 0030-4018 (74) 90024-8 .
- ^ JN Gannaway, CJR Sheppard: Second-harmonic imaging in the scanning optical microscope . In: Optical and Quantum Electronics . tape 10 , no. 5 , September 1978, p. 435-439 , doi : 10.1007 / BF00620308 .
- ↑ Shmuel Roth, Isaac Freund: Optical second-harmonic scattering in rat-tail tendon . In: Biopolymers . tape 20 , no. 6 , 1981, pp. 1271-1290 , doi : 10.1002 / bip.1981.360200613 .
- ^ I. Freund, M. Deutsch, A. Speaker: Connective tissue polarity. Optical second-harmonic microscopy, crossed-beam summation, and small-angle scattering in rat-tail tendon . In: Biophys. J. Band 50 , no. 4 , October 1986, p. 693-712 , doi : 10.1016 / S0006-3495 (86) 83510-X , PMID 3779007 , PMC 1329848 (free full text).
- ↑ Kenneth W. Dunn, Ruben M. Sandoval, Katherine J. Kelly, Pierre C. Dagher, George A. Tanner, Simon J. Atkinson, Robert L. Bacallao, Bruce A. Molitoris: Functional studies of the kidney of living animals using multicolor two-photon microscopy . In: Am. J. Physiol., Cell Physiol. tape 283 , no. 3 , 2002, p. C905 – C916 , doi : 10.1152 / ajpcell.00159.2002 , PMID 12176747 ( archived on October 29, 2013 [PDF]).
- ↑ Karel Svoboda, Ryohei Yasuda: Principles of two-photon excitation microscopy and its applications to neuroscience . In: Neuron . tape 50 , no. 6 , June 2006, p. 823-839 , doi : 10.1016 / j.neuron.2006.05.019 , PMID 16772166 .
- ↑ Stephanie Alexander, Gudrun E. Koehl, Markus Hirschberg, Edward K. Geissler, Peter Friedl: Dynamic imaging of cancer growth and invasion: a modified skin-fold chamber model . In: Histochem. Cell Biol. Volume 130 , no. 6 , December 2008, p. 1147-1154 , doi : 10.1007 / s00418-008-0529-1 , PMID 18987875 .
- ^ John A. Scherschel, Michael Rubar: Cardiovascular imaging using two-photon microscopy . In: Microsc. Microanal. tape 14 , no. 6 , December 2008, p. 492-506 , doi : 10.1017 / S1431927608080835 , PMID 18986603 .
- ^ I. Ben-Oren, G. Peleg, A. Lewis, B. Minke, L. Loew: Infrared nonlinear optical measurements of membrane potential in photoreceptor cells . In: Biophys. J. Band 71 , no. 3 , September 1996, p. 1616–1620 , doi : 10.1016 / S0006-3495 (96) 79365-7 , PMID 8874036 , PMC 1233629 (free full text).
- ↑ L. Moreaux, O. Sandre, J. Mertz: Membrane imaging by second-harmonic generation microscopy . In: J. Opt. Soc. At the. B . tape 17 , 2000, pp. 1685-1694 , doi : 10.1364 / JOSAB.17.001685 .
- ^ Paul J. Campagnola, Leslie M. Loew: Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms . In: Nat. Biotechnol. tape 21 , no. 11 , November 2003, p. 1356-1360 , doi : 10.1038 / nbt894 , PMID 14595363 .
- ^ Paul J. Campagnola, Mei-de Wei, Aaron Lewis, Leslie M. Loew: High-resolution nonlinear optical imaging of live cells by second harmonic generation . In: Biophys. J. Band 77 , no. 6 , December 1999, pp. 3341–3349 , doi : 10.1016 / S0006-3495 (99) 77165-1 , PMID 10585956 , PMC 1300605 (free full text).
- ↑ Cho-Shuen Hsieh, Shee-Uan Chen, Yen-Wei Lee, Yu-Shih Yang, and Chi-Kuang Sun: Higher harmonic generation microscopy of in vitro cultured mammal oocytes and embryos . In: Opt Express . tape 16 , no. July 15 , 2008, p. 11574-11588 , PMID 18648479 .






