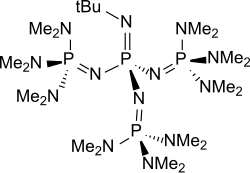
P 4 - t -Bu
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| The methyl groups (CH 3 groups) are given as Me | ||||||||||||||||
| General | ||||||||||||||||
| Surname | P 4 - t -Bu | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 22 H 63 N 13 P 4 | |||||||||||||||
| Brief description |
colorless crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 633.86 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
237 ° C with decomposition |
|||||||||||||||
| solubility |
Easily soluble in non-polar solvents such as tetrahydrofuran , diethyl ether , n-hexane , benzene and toluene , as well as in protic solvents with protonation |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
P 4 - t -Bu is an easily accessible representative from the group of neutral, peralkylated sterically hindered polyaminophosphazenes, which are extremely strong but only very weak nucleophilic bases . P 4 - t -Bu can also be understood as a tetrameric triaminoiminophosphorane with the basic structure (H 2 N) 3 P = NH. The homologous series of P 1 - to P 7 -Polyaminophosphazenen of the general formula with preferably methyl groups as R 1 , a methyl group or tert. Butyl group as R 2 and even-numbered x between 0 and 6 (P 4 - t -Bu: R 1 = Me, R 2 = t -Bu and x = 3) has been developed through the work of Reinhard Schwesinger; the phosphazene bases obtained are therefore also referred to as Schwesinger superbases .
Occurrence and representation
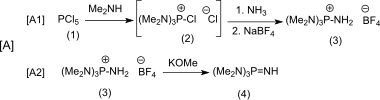
The convergent synthesis of P 4 - t -Bu starts from phosphorus pentachloride (1) and leads in branch [A] first via [A1] via the non-isolated chlorine (dimethylamino) phosphonium chloride (2) to the easily characterizable aminotris (dimethylamino) phosphonium tetrafluoroborate ( 3) and further via [A2] to the liquid Iminotris (dimethylamino) phosphorane (4)
and in branch [B] with phosphorus pentachloride and tert -butylammonium chloride to tert -butylphosphorimide trichloride (5)
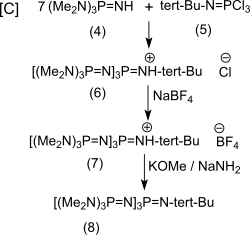
The reaction [C] of excess (4) with (5) gives the hydrochloride of the target product P 4 - t -Bu (6) with 93% yield ,
which is also converted into the tetrafluoroborate salt (7), from which the free base (8) can be obtained almost quantitatively with potassium methoxide / sodium amide or with potassium amide in liquid ammonia . The conversion of the hygroscopic and readily water-soluble hydrochlorides and the liquid free bases into the solid tetrafluoroborates, which are sparingly soluble in water, simplifies the handling of the substances considerably.
The relatively uncomplicated convergent synthesis with easily accessible reactants and very good yields of the intermediates make P 4 - t -Bu an interesting phosphazene superbase.
properties
With an extrapolated pK a value of 42.1 in acetonitrile, P 4 - t -Bu is one of the strongest neutral nitrogen bases and 18 orders of magnitude more basic than the strong base DBU with a pK a value of 24.3. The compound is very good in non-polar solvents, such as. B. hexane, toluene or tetrahydrofuran and is usually available as a 0.8 to 1 molar solution in hexane. Protonation even in weakly acidic media produces the extremely delocalized and soft cation P 4 - t -Bu-H cation and, in addition to a very strong solubilization effect, also causes an extreme acceleration of addition reactions even at temperatures below −78 ° C.
P 4 - t -Bu owes its extraordinarily high basicity with low nucleophilicity to its very high steric hindrance and the participation of many donor groups in the conjugation in the spatially demanding structure of the cation formed by protonation.
The base P 4 - t -Bu is an extremely hygroscopic solid that is thermally stable up to 120 ° C and chemically stable to (dry) oxygen and bases. Traces of water and protic impurities can be removed by adding bromoethane . The base is both very hydrophilic and very lipophilic and can be easily and almost completely recovered from reaction mixtures via the formation of the sparingly soluble tetrafluoroborate salt.
Because of its extremely weak Lewis basicity , the cation of P 4 - t -Bu suppresses typical side reactions of organometallic compounds, such as. B. Aldol condensations , as they can be caused by lithium amides such as lithium diisopropylamide (LDA).
Applications
The neutral superbase P 4 - t -Bu is superior to ionic bases if they are sensitive to oxidation or side reactions, such as B. acylation , cause solubility problems or Lewis acid -catalyzed side reactions cause such. B. aldol reactions , epoxy ring openings, etc.
The dehydrohalogenation of n-alkyl bromides, such as. B. 1-bromooctane with P 4 - t -Bu gives 1-octene in almost quantitative yield (96%) under mild conditions compared to the system potassium tert-butoxide / 18-crown-6 with only 75% yield.
Alkylation reactions on weakly acidic methylene groups , e.g. B. in the case of carboxylic acid esters or nitriles , proceed with high yield and selectivity. For example, when 8-phenylmenthylphenyl acetate is reacted with iodoethane in the presence of P 4 - t -Bu, only the monoethyl derivative in the Z configuration (95%) is obtained in 95% yield.
1,2-ethanedinitrile reacts with iodoethane in the presence of P 4 - t -Bu to give the tetraethyl derivative in 98% yield, without causing a Thorpe-Ziegler reaction with the formation of a cyclic α-ketonitrile.
Trifluoromethylation of ketones , such as. B. Benzophenone is also possible with the very inert fluoroform (HFC-23) in the presence of P 4 - t -Bu and tris (trimethylsilyl) amine at room temperature in good yields of up to 84%.
Intramolecular cyclization of ortho - alkynyl phenyl ethers leads to substituted benzofurans in the presence of P 4 - t -Bu under mild conditions without metal catalysts .
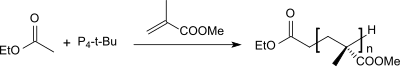
The extreme basicity of P 4 - t -Bu suggested early on that this superbase should be suitable as an initiator for anionic polymerization . From methyl methacrylate with the ethyl acetate / P 4 - t -Bu initiator system, polymethyl methacrylate (PMMA) with narrow polydispersity and molecular weights of up to 40,000 g · mol −1 could be obtained in the solvent THF .
Anionic polymerization of ethylene oxide with the initiator system n-butyllithium / P 4 - t -Bu provides defined polyethylene oxides with low polydispersity.
Cyclic siloxanes , such as. B. hexamethylcyclotrisiloxane or decamethylcyclopentasiloxane can also be polymerized with catalytic amounts of P 4 - t -Bu and water or silanols as initiator with good molar mass control to give thermally very stable polysiloxanes with decomposition temperatures> 450 ° C. Because of its extreme basicity, P 4 - t -Bu avidly absorbs water and carbon dioxide , both of which, however, inhibit anionic polymerization. Heating to temperatures> 100 ° C to remove CO 2 and water starts the anionic polymerization again.
The extreme hygroscopicity of the phosphazene base P 4 - t -Bu as a substance and in solutions requires enormous expenditure in terms of storage and handling and stands in the way of its wider use.
Individual evidence
- ↑ a b c d e f R. Schwesinger et al .: Extremely strong, uncharged auxiliary bases; Monomeric and polymer-supported polyaminophosphazenes (P2-P5) . In: Liebigs Ann. Chem. Band 7 , 1996, pp. 1055-10081 , doi : 10.1002 / jlac.199619960705 .
- ↑ a b c d R. Schwesinger, Y. Kondo: Phosphazene Base P 4 - t -Bu . In: e-EROS Encyclopedia of Reagents for Organic Synthesis . 2010, doi : 10.1002 / 047084289X.rp150.pub2 .
- ↑ a b data sheet Phosphazene base P 4 -t-Bu solution, ~ 0.8 M in hexane from Sigma-Aldrich , accessed on December 29, 2016 ( PDF ).
- ↑ R. Schwesinger et al .: How strong and how hindered can uncharged phosphazenes be? In: Angew. Chem. Band 105 , no. 9 , 1993, pp. 1420-1422 , doi : 10.1002 / anie.19931050940 .
- ↑ a b Patent US6353075B1 : Polymerization of siloxanes. Applied December 9, 1999 , published March 5, 2002 , Applicant: Dow Corning Ltd., Inventor: P. Hupfield, A. Surgenor, R. Taylor.
- ↑ J. Saame et al .: Experimental basicities of superbasic phosphonium ylides and phosphazenes . In: J. Org. Chem. Band 81 , no. 17 , 2016, p. 7349-7361 , doi : 10.1021 / acs.joc.6b00872 .
- ↑ ED Nacsa, TH Lambert: Higher-order cyclopropenimine super bases. Direct neutral Bronsted base catalyzed Michael reactions with α-aryl esters . In: J. Am. Chem. Soc. tape 137 , no. 32 , 2015, p. 10246-10253 , doi : 10.1021 / jacs.5b05033 .
- ↑ V. Gupta: New synthetic methods for biologically active aromatic heterocycles . Ed .: Iowa State University. Ames, Iowa 2010 ( online ).
- ↑ Patent EP0921128B1 : Process of preparing iminotris (dimethylamino) phosphorane. Filed on December 3, 1998 , published on September 25, 2002 , Applicant: Mitsui Chemicals, Inc., inventors T. nobori et al
- ^ R. Schwesinger, J. Willaredt, H. Schlemper, M. Keller, D. Schmitt, H. Fritz: Novel, Very Strong, Uncharged Auxiliary Bases; Design and Synthesis of Monomeric and Polymer-Bound Triaminoiminophosphorane Bases of Broadly Varied Steric Demand . In: Chem. Ber. tape 127 , no. 12 , 1994, pp. 2435-2454 , doi : 10.1002 / cber.199441271215 .
- ^ A b Strong and Hindered Bases in Organic Syntheses. (PDF; 1.2 MB) In: sigmaaldrich.com. Sigma-Aldrich, accessed December 20, 2016 .
- ↑ a b T. Pietzonka, D. Seebach: The P4-phosphazene base as part of a metal-free initiator system for the anionic polymerization of methacrylic acid methyl ester . In: Angew. Chem. Band 105 , no. 5 , 1993, p. 741-742 , doi : 10.1002 / anie.19931050514 .
- ↑ R. Schwesinger, H. Schlemper: Peralkylated polyaminophosphazene - extremely strong, neutral nitrogen bases . In: Angew. Chem. Band 99 , no. 11 , 1987, pp. 1212-1214 , doi : 10.1002 / anie.19870991134 .
- ↑ A. Solladié-Cavallo, AG Csaky, I. Gantz, J. Suffert: Diastereoselective Alkylation of 8-Phenylmenthyl Phenylacetate: Aggregated Lithium Enolate versus "Naked" Enolate . In: J. Org. Chem. Band 59 , no. 18 , 1994, p. 5343-5346 , doi : 10.1021 / jo00097a041 .
- ↑ S. Okusu, K. Hirano, E. Tokunaga, N. Shibata: Organocatalyzed trifluoromethylation of ketones and sulfonyl fluorides by fluoroform under a super base system . In: ChemistryOpen . tape 4 , 2015, p. 581-585 , doi : 10.1002 / open.201500160 .
- ↑ C. Kanazawa, K. Goto, M. Terada: Phosphazene base-catalyzed intramolecular cyclization for efficient synthesis of benzofurans via carbon-carbon bond formation . In: Chem. Commun. 2009, p. 5248-5250 , doi : 10.1039 / B913588J .
- ↑ B. Eßwein, M. Möller: Polymerization of ethylene oxide with alkyllithium compounds and the phosphazene base "t Bu-P4" . In: Angew. Chem. Band 108 , no. 6 , 1996, pp. 703-705 , doi : 10.1002 / anie.19961080620 .
- ↑ PC Hupfield, RG Taylor: Ring-opening polymerization of siloxanes using phosphazene base catalysts . In: J. Inorg. Organomet. Polym. Mater. tape 9 , no. 1 , 1999, p. 17-34 , doi : 10.1023 / A: 1021429320083 .




![{\ displaystyle \ mathrm {[(R_ {2} ^ {1} N) _ {3} P = N -] _ {x} - (R_ {2} ^ {1} N) _ {3-x} P = NR ^ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/00b61ce1df0315096a02b5c926276a98bab411d8)