Pentafluorophenylammonium triflate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Pentafluorophenylammonium triflate | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 7 H 3 F 8 NO 3 S | ||||||||||||||||||
| Brief description |
white to pale red crystal powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 333.15 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
211.5 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
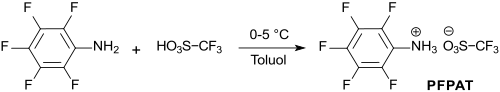
Pentafluorophenylammonium triflate is the salt of pentafluoroaniline and trifluoromethanesulfonic acid , which can be used as an acidic and metal-free catalyst for the formation of carboxylic acid esters , thioesters and lactones , as well as for transesterifications under comparatively mild conditions with high efficiency. In the Ritter reaction of nitriles to amides , high yields are achieved with PFPAT under moderate conditions.
Occurrence and representation
Pentafluorophenylammonium triflate is obtained in 95% yield when stoichiometric amounts of trifluoromethanesulfonic acid TfOH are added to 2,3,4,5,6-pentafluoroaniline (from hexafluorobenzene and sodium amide in liquid ammonia or with ammonia in ethanol in a sealed tube ).
properties
In its pure form, pentafluorophenylammonium triflate is a white, water-soluble crystal powder.
Applications
The easily accessible, strong Brønsted acid pentafluorophenylammonium triflate is said to have a number of advantages for acid-catalyzed reactions as a “green catalyst” according to literature. Mention is made of a favorable price, low toxicity, small amounts used, mild reaction conditions, easy implementation, e.g. B. solvent-free one-pot processes, short reaction times, air and water resistance, high yields, gentle on N-containing substrates and many functional groups, easy processing and good recyclability.
With catalytic amounts (1-10 mol%) of pentafluorophenylammonium triflate, carboxylic acid esters , thioesters and macrocyclic lactones can be formed and transesterifications can be catalyzed in toluene as a solvent at elevated temperatures (40-110 ° C) .
Yields of over 90% are usually achieved.
The seventeen-membered lactone 16-hexadecanolide is formed from 16-hydroxypalmitic acid under dilution conditions (10 mmolar in toluene) in 87% yield, along with 10% of the circular diester.
Even without a solvent, the acylation of alcohols, phenols and amines with acetic anhydride with PFPAT catalysis (10 mol%) usually takes place at room temperature and within <60 minutes in yields of over 90%.
Thus, 2-phenylethyl acetate is formed from 2-phenylethanol and acetic anhydride at room temperature within 20 minutes in 97% yield.
Pentafluorophenylammonium triflate catalyzes the C- acylation of enol silyl ethers with carboxylic acid chlorides to give β- diketones in good to very good yields (62–92%).
PFPAT can also be used to catalyze the synthesis of α-aminophosphonates from aldehydes, amines and dimethyl phosphite analogous to a standard synthesis of the total herbicide glyphosate with yields of 80–95%.
Instead of sulfuric acid (or an H 2 SO 4 / acetic acid mixture), pentafluorophenylammonium triflate can be used as an effective catalyst in the Ritter reaction .
Addition of catalytic amounts of PFPAT to a mixture of tert- butanol or tert-butyl acetate and acrylonitrile gives N-tert-butyl acrylamide in 92% yield .
The catalysis of the polycondensation of lactic acid with only 0.1 mol% PFPAT in aromatic solvents provides polylactic acid with average molar masses of up to> 100,000 g / mol.
Individual evidence
- ↑ a b Entry on Pentafluoranilinium trifluoromethanesulfonate at TCI Europe, accessed on May 28, 2019.
- ↑ a b Patent EP2028209A1 : Organic acid catalyst for polylactic acid synthesis. Filed June 12, 2007 , published February 25, 2009 , Applicants: National University Corp., Kyoto Institute of Technology, Inventors: A. Abiko, H. Iwahashi.
- ↑ a b c d e T. Funatomi, K. Wakasugi, T. Misaki, Y. Tanabe: Pentafluorophenylammonium triflate (PFPAT): an efficient, practical, and cost-effective catalyst for esterification, thioesterification, transesterification, and macrolactone formation . In: Green Chem. Band 8 , no. 12 , 2006, p. 1022-1027 , doi : 10.1039 / B609181B .
- ↑ a b MSDS Synquest Laboratories. January 12, 2017, accessed June 26, 2019 .
- ↑ a b c S. Khaksar, E. Fattahi, E. Fattahi: Organocatalytic synthesis of amides from nitriles via the Ritter reaction . In: Tetrahedron Lett. tape 52 , no. 45 , 2011, p. 5943-5946 , doi : 10.1016 / j.tetlet.2011.08.121 .
- ↑ EJ Forbes, RD Richardson, JC Tatlow: Pentafluoroaniline . In: Chemistry & Industry . 1958, p. 630 .
- ↑ GM Brooke, J. Burdon, M. Stacey, JC Tatlow: 350. Aromatic perfluoro compounds. Part IV. The reaction of aromatic polyfluoro compounds with nitrogen-containing bases . In: J. Chem. Soc. 1960, p. 1768-1771 , doi : 10.1039 / jr9600001768 .
- ↑ S. Khaksar, H. Zakeri: Pentafluorophenylammonium triflate as a mild and new organocatalyst for acylation of alcohols, phenols, and amines under solvent-free condition . In: Comb. Chem. High Throughput Screen. tape 15 , no. 7 , 2012, p. 576-579 , doi : 10.2174 / 138620712801619203 .
- ↑ A. Iida, J. Osada, R. Nagase, T. Misaki, Y. Tanabe: Mild and efficient pentafluorophenylammonium triflate (PFPAT) -catalyzed C-acylations of enol silyl ethers or ketene silyl (thio) acetals with acid chlorides . In: Org. Lett. tape 9 , no. 10 , 2007, p. 1859-1862 , doi : 10.1021 / ol070191b .
- ↑ F. Malamiri, S. Khaksar: Pentafluorophenylammonium triflate (PFPAT): A new organocatalyst for the one-pot three-component synthesis of α-aminophosphonates . In: J. Chem. Sci. tape 126 , no. 3 , 2014, p. 807-811 , doi : 10.1007 / s12039-014-0636-6 .