Boron nitride
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| __ B 3+ __ N 3− | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Boron nitride | |||||||||||||||
| Ratio formula | BN | |||||||||||||||
| Brief description |
white, odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 24.83 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.25 g cm −3 (α-BN, hexagonal) |
|||||||||||||||
| Melting point |
2967 ° C |
|||||||||||||||
| solubility |
almost insoluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−254.4 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Boron nitride , chemical formula BN, is a boron - nitrogen compound that occurs in three modifications (α, β, γ). According to their crystal structure, they are also known as hexagonal, cubic or wurtzitic boron nitride (h-BN, c-BN, w-BN). The cubic β-BN is stable under normal conditions, the other two modifications are metastable, and amorphous boron nitride does not crystallize either. The hexagonal α-BN is stable above 1200 ° C up to the melting point, the wurtzitic γ-BN only at very high pressures above approx. 10 GPa. Of course, the metastable hexagonal α-boron nitride occurs exclusively. The three modifications are analogous to those of carbon, which is isoelectronic with boron nitride . Hexagonal boron nitride can be assigned to graphite, cubic to diamond, and wurtzitic to Lonsdaleite . Correspondingly, boron nitride is a high polymer, in the case of the hexagonal modification with very low hardness and sliding properties, in the cubic case with extremely high hardness. Cubic β-boron nitride was first synthesized by Robert Wentorf in 1957 and was the hardest known material at the time after diamonds. In 1969, CBN (cubic crystalline boron nitride) was brought onto the market by the General Electric company under the name " BORAZON "; the price then exceeded the gold price . Commercial production of CBN has only been established since the early 1990s.
properties
CBN is one of the hardest materials. Under normal conditions, boron nitride has a Knoop hardness of approx. 48 GPa (48,000 N / mm²), compared with diamond between 70 and 100 GPa.
When used appropriately, tools made of CBN wear out much more slowly than other cutting materials . On the one hand, this enables higher form and dimensional accuracy to be achieved, and on the other hand, very hard materials (steel up to 70 HRC ) can be processed reliably. However, due to its high hardness, CBN is very brittle, which puts its suitability for machining with interrupted cuts into perspective.
Similar to diamond, CBN has a high thermal conductivity , namely five times the thermal conductivity of copper, whereby the heat z. B. is taken up by the grinding wheel during grinding and can be released to the coolant or to the environment. The workpiece heats up far less than when sanding with corundum , so that the structure of the edge zone is less affected. The relatively high grinding temperatures do not attack CBN chemically when machining iron , nickel or cobalt .
CBN tools can be used both with and without cooling lubricant.
While diamond suffers a massive loss of hardness at around 700 ° C, the hardness of CBN remains almost unchanged at more than 1000 ° C. Diamond can be ground with CBN under the action of heat.
Crystal structures
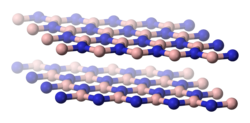
Structurally, α-boron nitride can be compared to graphite . It also consists of layers of a planar, hexagonal honeycomb structure in which the B and N atoms occur alternately. In contrast to graphite, the hexagons of the individual layers are arranged in a congruent manner, so that an N atom can be found below and above each B atom (and vice versa). The physical properties of α-BN and graphite are very similar. Their densities are practically identical and both have a very high melting point. They also feel like talc on the skin when rubbed. There is, however, a difference in terms of electrical conductivity. α-BN only conducts electricity at very high temperatures. Before that, the p π -p π backbonding electrons are preferably located near nitrogen due to the EN difference. The π-electrons are therefore not free to move and a current transmission is not possible.
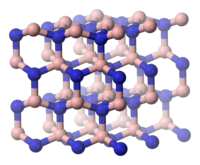
The cubic β-boron nitride, also CBN ( English Cubic Boron Nitride ), is in the cubic sphalerite structure, the aristotype of which is the diamond structure . The γ-shape, on the other hand, corresponds to the hexagonal wurtzite structure, the aristotype of which is the hexagonal diamond Lonsdaleite . However, it is metastable to the β form. The more than 50% increased density of the diamond-like forms of boron nitride compared to the graphite-like α-form can be explained by the fact that the layer spacing is reduced by approx. 30%.
 |
 |
|
| α-BN, hexagonal | β-BN, cubic structure | BN, hexagonal crystal system or wurtzite structure |
Manufacturing
To produce hexagonal α-boron nitride, boron trioxide B 2 O 3 can be reacted with elemental nitrogen N 2 . This reaction is carried out at high temperatures with the catalysis of calcium phosphate Ca 3 (PO 4 ) 2 . Instead of nitrogen, ammonia NH 3 or an ammonium compound can also be used, and instead of boron oxide, a boron halide can also be used. This creates the (colorless) α-modification.
Similar to diamond from graphite, β-boron nitride (CBN) can be produced from α-BN at high temperatures (1500–2200 ° C) and high pressure (50–90 kbar). Lithium nitride (Li 3 N) can be used as a catalyst . At even higher pressures, the γ-shape arises. However, since the return to normal conditions leads through the phase region of the β-BN, there is a risk of conversion if this process is not sufficiently fast. The synthesis of γ-BN is therefore also carried out at lower temperatures in order to be able to reach the metastable state more quickly.
use

From a technical point of view, β-boron nitride (CBN) is primarily used as an abrasive and as a cutting material for indexable inserts for machining steel , because - unlike diamonds - it cannot release carbon to steel when exposed to temperature. It is also used for surface coating for the same reason.
The graphite-like hexagonal modification (α-boron nitride) is used as a lubricant (“inorganic” or “white graphite”). In contrast to graphite, the coefficient of friction of hexagonal boron nitride remains stable up to over 1000 ° C, which is why it is very suitable as a high-temperature solid lubricant under vacuum. At high temperature (1400–1800 ° C) and high pressure (> 6 GPa), the hexagonal modification is converted into the cubic modification, analogous to the conversion of graphite into diamond. Both boron nitride modifications are white and do not conduct electricity at low and moderate temperatures.
Although hexagonal boron nitride was used in cosmetics as early as 1940, it did not gain acceptance until the 1990s, after manufacturing costs had fallen sharply. The high coverage and the graphite-like texture, which is advantageous for application, are the decisive properties for use in make-up.
A porous sponge made of boron nitride is able to absorb about 33 times its own weight in oil and organic solvents. After the absorbed liquids have been burned or evaporated, the filter sponge can be recovered unchanged. This property could be used for water treatment or purification.
Hexagonal boron nitride is being investigated as a potential material for (UV) light-emitting diodes because of its interesting properties as a III-V compound semiconductor (band gap 5.8 eV, high mobility of electrons and holes).
Use as an abrasive
CBN wheels are used for grinding:
- hardened high speed steels (HSS)
- high-alloy tool steels with min. 55 HRC
- case-hardened steels
- Iron-based powder coatings
- Chilled cast iron
- Soft steel grades in certain applications
- Stellite
- Nickel-based superalloys
Grinding speed
The optimal cutting speed depends on various factors:
- Type of grinding (round, flat, pendulum, deep grinding, etc.)
- Cooling (oil, dry grinding)
- Machine (stability, spindle speed)
With CBN, under optimal conditions, you can work with high-speed grinding instead of the normal 30–60 m / s for wet grinding, or the 15–20 m / s at much higher speeds. For this, however, the machines must be designed to be appropriately stable, and the oil cooling must be carried out with the right nozzles and sufficient pressure.
Cool
When grinding with CBN, pure grinding oil is used for cooling. The generation of heat is avoided by the oil and its lubricating effect. At the same time, the energy consumption is significantly reduced. The service life is increased by three times compared to other cooling media. It is quite possible and is also used to cool with an oil-water emulsion. However, not only is the service life shorter, but also the contamination of the machine with emulsion compared to pure grinding oil is significantly higher.
However, a distinction is made between discs that are specially designed for wet grinding and should then only be used in dry grinding with reduced speed and infeed only in exceptional cases. Discs designed for dry grinding can also be used for wet grinding; However, in dry sanding, lower contact pressures and infeeds should be used.
Ties
During CBN grinding, the grains are coated with a thin metal layer made of nickel or copper. As a result, the grain is optimally held in the bond and the resulting grinding heat is conducted into the bond. A distinction is made between different types of binding according to the binding material.
Resin bonds
Over 50% of all grinding tasks can be accomplished with synthetic resin bonds, as many bond variants and high removal rates are possible. In addition, the wheel has a great grip and causes low grinding pressures and low temperatures. They are suitable for both dry and wet grinding.
Body material:
- aluminum
- Aluminum resin
- Graphite resin
- Some manufacturers also use ceramics as the base material if they are very fine-grained (<B25 = 30–20 µm) discs.
Metal bonds
Metal bonds have very high grain holding forces and are therefore mainly used in wet grinding. Because of their high wear resistance, they are used in particular for profile disks with high profile retention. In terms of machining performance, however, they are inferior to plastic bonds.
Body material:
- steel
- bronze
Galvanic bonds
Usually only the grain layer is held, the grains being embedded in a nickel bond and approx. 30–50% protruding from the bond. This creates a very good grip with a very high grinding performance. The total service life is very short, however, as it ends when the lining height is worn out.
Body material:
- steel
- Aluminum (the aluminum base body material is first copper-plated before coating with CBN, because nickel does not adhere well or does not adhere to aluminum)
Ceramic bonds
The porous and profilable bond is suitable for long-chipping materials. The result is low grinding forces, high surface quality, high cutting performance and dressing options.
Body material:
- steel
- aluminum
- Ceramic (so-called "composite panes")
- Carbon fiber
When selecting the binding, it is important that the grain is kept in the binding as long as it still has edges. However, if the grains are blunt, they have to break away from the bond. If the grit holding force is too high, the grinding pressure and the temperature increase: the wheel becomes clogged, smeared and loses its removal rate.
Guidelines
machinery
The machines that are used for CBN grinding should be extremely sturdy and have perfectly running grinding spindles and wheel holders. The guides must work free of play, the table must move smoothly and the entire machine must be set up free of vibrations. In addition, the motor output must be dimensioned in such a way that higher cutting speeds can be used and that there is no significant drop in speed with larger infeeds.
Discs
Discs must be ground as precisely as possible so that they wear evenly. One possibility for large disks is to send a flange and a suitable grinding or balancing mandrel to the manufacturer so that the disk can be ground with them and the run-out deviation is as low as possible. The disc must then remain on the holder until it is completely worn out in order to avoid possible runout errors.
Polycrystalline cubic boron nitride
P olykristallines k ubisches B ornitrid (PKB / English PCBN ) is a synthetic composite material of cubic boron nitride (cBN) with ceramic binder phase. For the production of PCBN, cBN micro-grains are synthesized from hexagonal boron nitride at high temperatures and pressures . These cBN particles are then sorted and characterized before they go through a second synthesis process, which is carried out with the addition of a ceramic binder material. PCBN is available in different PCBN-binder ratios and formats.
PKB is named as a cutting material according to ISO standard BN , but the designations CBN or PCBN are also often found.
use
Polycrystalline Cubic Boron Nitride is widely used in machining a wide variety of hard and / or abrasive FE workpiece materials. PCBN is chemically inert up to high temperatures and, unlike PCD , does not react with the iron in ferrous materials. Typical parts that are machined with PCBN are e.g. B. Brake discs , engine blocks , cylinder liners, brake drums , flywheels , valve seats and guides, machine parts, gears, pressed and stamped parts, etc.
Typical workpiece materials are:
- Tool steel for hot / cold work (45-65 HRC)
- Case-hardened steel (45-65 HRC)
- High speed steel (45-65 HRC)
- Bearing steel (45-65 HRC)
- Sintered iron (45-65 HRC)
- Weld-on alloys (> 35 HRC)
- Gray cast iron (200–280 HBN)
Pyrolytic boron nitride
Very closely related to β-boron nitride is pyrolytic boron nitride, which is abbreviated as pBN or PBN. Because of the low outgassing even at high temperatures, crucibles made of pBN are used as effusion cells in molecular beam epitaxy .
Individual evidence
- ↑ a b data sheet boron nitride from AlfaAesar, accessed on February 2, 2010 ( PDF )(JavaScript required) .
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 .
- ↑ David R. Lide: CRC Handbook of Chemistry and Physics . 90th edition. Taylor & Francis, 2009, ISBN 978-1-4200-9084-0 .
- ↑ a b data sheet boron nitride from Sigma-Aldrich , accessed on March 14, 2011 ( PDF ).
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-6.
- ↑ Borazon-CNB. ( Memento from September 30, 2011 in the Internet Archive )
- ↑ Georg Brauer , with the collaboration of Marianne Baudler a . a. (Ed.): Handbook of Preparative Inorganic Chemistry . 3rd, revised edition. tape I . Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , pp. 806 .
- ↑ Martin Engler, Christoph Lesniak, Ralf Damasch, Bernd Ruisinger, Jens Eichler: Hexagonal Boron Nitride (hBN) - Applications from Metallurgy to Cosmetics . In: Ceramic forum international, reports of the German Ceramic Society . tape 84 , 2007, ISSN 0173-9913 , p. E49-E53 ( PDF ).
- ↑ Lars Fischer: Boron nitride sponge removes oil from water. Report at Spektrum.de from May 2, 2013.
- ↑ Weiwei Lei, David Portehault, Dan Liu, Si Qin & Ying Chen: Porous boron nitride nanosheets for effective water cleaning. In: Nature Communications. 4 (1777) April 30, 2013; doi: 10.1038 / ncomms2818 .
- ↑ Katrin Sedlmeier: Water treatment with UV LEDs. (PDF) TU-Berlin , 2008, pp. 9–10 , accessed on June 6, 2015 .






